Improving postoperative pain management after video-assisted thoracic surgery lung resection contributes to enhanced recovery, but guidelines are still lacking
We read with great interest the article by Ghee et al. (1). We appreciate the efforts dedicated by the authors to the design of one of the few randomized controlled trials on this topic.
The authors hypothesized that postoperative subpleural catheter bupivacaine infusion after video-assisted thoracic surgery (VATS) for lobectomy and wedge resection would improve pain and reduce postoperative pain medication use. To this aim, they randomized 86 patients to receive either intraoperative incision site injection of 0.25% bupivacaine or subpleural tunneled catheter (On-Q: Halyard Medical, Alpharetta, GA, USA) and 0.125% bupivacaine continuous infusion 2 mL/h. The patients underwent thoracoscopic surgery with two incisions. The authors found no difference in opioids, acetaminophen or non-steroidal anti-inflammatory drugs (NSAID) use between the two groups. Patients in the subpleural group reported greater NSAID usage on postoperative days 4 to 7, likely due to irritation at the catheter insertion sites. Length of hospital stay did not differ between the two groups.
The authors concluded that no benefits are associated with the use of local anesthetic infusion through subpleural pain catheters during the postoperative period after thoracoscopic wedge resection or lobectomy.
This paper is interesting because optimal postoperative analgesia after VATS lobectomy remains unclear; moreover, RCTs on this topic are rare. Most studies are retrospective or uncontrolled.
The authors well addressed the limitations of their study; there were several missing self-reported data, and the concentration of bupivacaine used was low, compared to other studies.
The authors’ conclusions match our clinical experience. We already used local anesthetic infusion through tunneled catheters after thoracotomy, but we abandoned this analgesic technique because it did not show any significant clinical benefit. We appreciate and agree with authors’ conclusions, and would like to add some considerations.
As mentioned by the authors, it is difficult to compare their study to those available in literature because of differences in technique and study design. The authors cited a paper by Wildgaard et al. (2), who used an intercostal catheter and found it to be effective in controlling postoperative pain. This study was uncontrolled, thus we cannot draw conclusions about the efficacy of this technique; however, patients were treated with bupivacaine 0.5%. A total of 15 mL (75 mg) of the local anesthetic were distributed in intercostal spaces from T3 to T8, followed by continuous 0.25% bupivacaine infusion at 6 mL/h (15 mg/h) through an epidural catheter positioned into the intercostal space. The first consideration we would like to make is that the 0.125% bupivacaine at 2 mL/h (2.5 mg/h, i.e., six times lower) infusion regimen used in this study may be too small to obtain success in controlling pain. Hence, this dose regimen cannot be compared to other studies that used doses similar to that used by Wildgaard et al. In a prospective, nonrandomized, comparative study of thoracic epidural analgesia (n30) vs. On-Q catheter local anesthetic infusion (n32), Ried et al. used 0.75% ropivacaine continuous infusion 5 mL/h (37.5 mg/h, i.e., 15 times higher) to treat postoperative pain after thoracic surgery (3). Hsieh et al., in a retrospective comparison between local anesthetic intercostal catheter infusion and single shot intercostal nerve analgesia in patients undergoing single port VATS, showed effectiveness of continuous single intercostal nerve block (4). Ten milliliters of 0.5% levobupivacaine (50 mg) were injected at the end of surgery into the subpleural space in both groups followed by 0.5% levobupivacaine infusion 2.5 mL/h only in the study group (12.5 mg/h, 5 times higher).
We understand that Ghee et al. chose this concentration to avoid the paresthesia associated with higher concentrations of bupivacaine, like 0.5%, while the 0.125% concentration showed, in their experience, the same efficacy without being associated to paresthesia. However, these are subjective experiences that received no confirmation in literature. It would have been more interesting to compare an infusion rate already used in clinical studies and showing definite effectiveness in anesthetizing intercostal nerves. The authors also state that their technique is similar to epidural placement, hence supporting that the local anesthetic infusion doses should be similar to those of epidural analgesia. However, this is subjective and not supported by literature; in fact, we failed to find any report that the two spaces share similar characteristics.
A second consideration regards chest tube removal. Table 1 of Ghee et al. shows that 28 out of 36 (77.7%) patients in the control group, and 30 out of 41 (73%) patients in the SPC group had tube removal within the first postoperative day (1). To our opinion, because patients underwent major thoracic surgery, chest tube removal timing seems very short, considering current clinical practice. It would be interesting to know which criteria the authors followed to safely remove the chest tubes, as the authors did not specify them in their otherwise remarkable paper.
Full table
A further consideration concerns the local infiltration of the incision site chosen for the control group. Post-thoracotomy pain mainly derives from crushing the intercostal neurovascular bundle during rib spreading and from the chest tube (5). Wound infiltration with local anesthetics provides only local analgesia surrounding the two incision sites and, possibly, the chest tube entry site. Most postoperative pain remains uncontrolled if intercostal nerves are not blocked. Both groups should have undergone intercostal nerve block followed in the study group by intercostal local anesthetic continuous infusion to obtain adequate and comparable pain control. In fact, to assess exclusively the efficacy of subpleural catheter-conducted local anesthetic infusion, the study and the control groups should have undergone the same procedure up to a point, with wound infiltration followed by continuous local anesthetic infusion. We do not know whether the catheter would have been more effective, had the experimental group been subjected to wound infiltration followed by anesthetic infusion. The information we get from the study is just that the two techniques (wound local anesthetic infiltration and continuous intercostal local anesthetic infusion without wound infiltration) show similar effectiveness.
The authors should be praised for their open approach and their intellectual honesty.
Pain after lobectomy may be extremely severe. Thoracotomy is considered one of the highest risk operations for chronic persistent postoperative pain (>3 months). The incidence of moderate or severe thoracic pain at 1 year following thoracotomy is between 11–30% and 3–5%, respectively (6-8). In most patients, post-thoracotomy pain is usually severe until 1-month postoperatively, then gradually decreases and disappears one year later (6,7). Thoracotomy is considered, along with limb amputation, to be the surgical procedure that elicits the highest risk of severe chronic postoperative pain (6), half of which definitely or possibly includes a neuropathic component (9).
Besides the development of chronic pain, uncontrolled post-thoracotomy pain may lead to pulmonary complications, i.e., hypoxia, atelectasia, and pneumonia (10,11), delay in mobilizing the patient, i.e., thrombosis and pulmonary embolism (12), and prolonged hospital stay. Because of rib spreading and intercostal suturing, thoracotomy is often associated with intercostal neurovascular bundle damage. In this light, VATS should allow a reduced traumatism to the intercostal nerve possibly leading to less postoperative pain and complications. Although literature supporting fast track recovery associated to VATS is scanty, a recent report showed that developing protocols to prevent factors that delay postoperative recovery or cause complications (Enhanced Recovery After Surgery—ERAS society) shortens hospital stay, costs, and opioid usage (13). Nevertheless, guidelines from the ERAS society in thoracic surgery are still lacking (14). To this aim, it is of paramount importance to identify which postoperative pain management is best.
While thoracic epidural anesthesia/analgesia and paravertebral blocks represent the gold standard for post-thoracotomy pain treatment (15), optimal postoperative analgesia after VATS lobectomy has not yet been established.
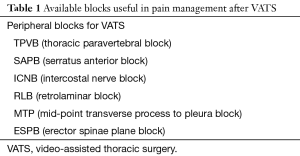
Currently, several analgesic options are available. ERAS protocols stress the importance of a multimodal drug regimen, using drugs with different analgesic mechanisms, associated with peripheral nerve blockade, to obtain the best pain relief and opioid sparing effect (14). Systemic opioids are associated with side effects, such as gastrointestinal dysfunction, and should be administered through a patient-controlled device (PCA), to account for interindividual pharmacokinetic and pharmacodynamic variability. Recently, pregabalin (16) and buprenorphine (17) have been suggested to reduce either postoperative hyperalgesia or unwanted side effects. Peripheral anesthetic blockades include paravertebral block, intercostal nerve local anesthetic infusion, and other anesthetic blocks (Table 1). Thoracic epidural catheters (TEA) are effective but may prolong hospital stay (18).
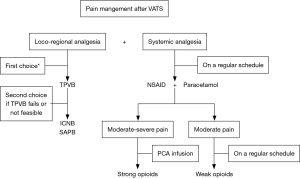
Actually, the thoracic paravertebral block (TPVB) is the technique that combines efficacy to a high safety profile, representing the first choice for pain management after VATS (19). Intercostal nerve block (ICNB) is an efficacious and easy to perform block and may represent a second choice to TPVB (15,20). Although serratus anterior plane block (SAPB) is used mainly for breast surgery, increasing evidence shows its analgesic efficacy in both thoracotomy (21) and VATS (22). Retrolaminar block (RLB) (23), mid-point transverse process to pleura block (MTP) (24), and erector spinae plane block (ESPB) (25) have still to show sufficient evidence to be recommended in VATS postoperative analgesia. Nevertheless, TEA should be considered in case of high-risk of thoracotomy conversion. The flowchart in Figure 1 briefly reports the approach suggested by Piccioni and Ragazzi in a recent report on pain management for VATS (26).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ghee CD, Fortes DL, Liu C, et al. A Randomized Controlled Trial of Continuous Subpleural Bupivacaine After Thoracoscopic Surgery. Semin Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Wildgaard K, Petersen RH, Hansen HJ, et al. Multimodal analgesic treatment in video-assisted thoracic surgery lobectomy using an intraoperative intercostal catheter. Eur J Cardiothorac Surg 2012;41:1072-7. [Crossref] [PubMed]
- Ried M, Schilling C, Potzger T, et al. Prospective, comparative study of the On-Q(R) PainBuster(R) postoperative pain relief system and thoracic epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2014;28:973-8. [Crossref] [PubMed]
- Hsieh MJ, Wang KC, Liu HP, et al. Management of acute postoperative pain with continuous intercostal nerve block after single port video-assisted thoracoscopic anatomic resection. J Thorac Dis 2016;8:3563-71. [Crossref] [PubMed]
- Andreetti C, Menna C, Ibrahim M, et al. Postoperative pain control: videothoracoscopic versus conservative mini-thoracotomic approach. Eur J Cardiothorac Surg 2014;46:907-12. [Crossref] [PubMed]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- Gotoda Y, Kambara N, Sakai T, et al. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain 2001;5:89-96. [Crossref] [PubMed]
- Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand 1999;43:563-7. [Crossref] [PubMed]
- Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231-43. [Crossref] [PubMed]
- Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg 1998;86:598-612. [Crossref] [PubMed]
- Berrisford RG, Sabanathan SS, Mearns AJ, et al. Pulmonary complications after lung resection: the effect of continuous extrapleural intercostal nerve block. Eur J Cardiothorac Surg 1990;4:407-10; discussion 411. [Crossref] [PubMed]
- Dentali F, Malato A, Ageno W, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2008;135:705-6. [Crossref] [PubMed]
- Martin L, Sarosiek B, Harrison M, et al. Implementing a Thoracic Enhanced Recovery Program: Lessons Learned In the First Year. Ann Thorac Surg 2018.
- Beverly A, Kaye AD, Ljungqvist O, et al. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol Clin 2017;35:e115-e43. [Crossref] [PubMed]
- Elmore B, Nguyen V, Blank R, et al. Pain Management Following Thoracic Surgery. Thorac Surg Clin 2015;25:393-409. [Crossref] [PubMed]
- Matsutani N, Dejima H, Nakayama T, et al. Impact of pregabalin on early phase post-thoracotomy pain compared with epidural analgesia. J Thorac Dis 2017;9:3766-73. [Crossref] [PubMed]
- Mercieri M, Palmisani S, De Blasi RA, et al. Low-dose buprenorphine infusion to prevent postoperative hyperalgesia in patients undergoing major lung surgery and remifentanil infusion: a double-blind, randomized, active-controlled trial. Br J Anaesth 2017;119:792-802. [Crossref] [PubMed]
- Gebhardt R, Mehran RJ, Soliz J, et al. Epidural versus ON-Q local anesthetic-infiltrating catheter for post-thoracotomy pain control. J Cardiothorac Vasc Anesth 2013;27:423-6. [Crossref] [PubMed]
- Kosinski S, Fryzlewicz E, Wilkojc M, et al. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther 2016;48:280-7. [PubMed]
- D'Andrilli A, Ibrahim M, Ciccone AM, et al. Intrapleural intercostal nerve block associated with mini-thoracotomy improves pain control after major lung resection. Eur J Cardiothorac Surg 2006;29:790-4. [Crossref] [PubMed]
- Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-Guided Serratus Anterior Plane Block Versus Thoracic Epidural Analgesia for Thoracotomy Pain. J Cardiothorac Vasc Anesth 2017;31:152-8. [Crossref] [PubMed]
- Okmen K, Metin Okmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med 2017. [Epub ahead of print]. [PubMed]
- Voscopoulos C, Palaniappan D, Zeballos J, et al. The ultrasound-guided retrolaminar block. Can J Anaesth 2013;60:888-95. [Crossref] [PubMed]
- Costache I, de Neumann L, Ramnanan CJ, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia 2017;72:1230-6. [Crossref] [PubMed]
- Rao Kadam V, Currie J. Ultrasound-guided continuous erector spinae plane block for postoperative analgesia in video-assisted thoracotomy. Anaesth Intensive Care 2018;46:243-5. [PubMed]
- Piccioni F, Ragazzi R. Anesthesia and analgesia: how does the role of anesthetists changes in the ERAS program for VATS lobectomy. J Vis Surg 2018;4:9. [Crossref] [PubMed]


