Intraoperative descending aortic dissection during aortic root replacement: successful management
Introduction
Intraoperative aortic dissection is a very rare complication during surgical cardiac intervention with occurrence frequencies of 0.06–0.23% (1-3). However, when it does occur it is uniformly a life-threatening complication with high rates of morbidity and mortality (3-5). Despite the potentially catastrophic outcomes of intraoperative aortic dissection, no clear guidelines exist regarding optimal management.
We present our surgical technique for a case of intraoperative acute type B aortic dissection (IABD) that occurred during an elective aortic root replacement procedure. We altered our surgical intervention slightly and subsequently managed the aortic dissection with medical therapy per the “complication specific” approach described previously (6-9). The therapy completely stabilized the descending dissection and at 6-week imaging follow-up no ischemic or neurological complications were evident.
Surgical techniques
Case presentation
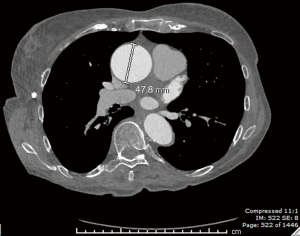
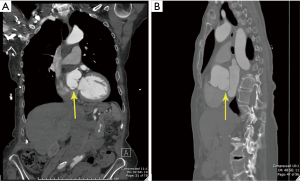
A 76-year-old hypertensive woman with bicuspid aortic valve leading to moderately severe aortic insufficiency presented with an enlarging ascending aortic aneurysm (from 4.4 to 4.8 cm over 6 months, Figure 1). The patient did not report any family history of aortic aneurysm, dissection or sudden death. Cardiac catheterization confirmed ascending aortic aneurysm and aortic insufficiency, with clean coronary arteries. Computer tomography (CT) confirmed an ascending aortic aneurysm of 4.8 cm with aortic root dilation. CT also revealed a focal outpouching of the non-coronary cusp of the sinus of Valsalva, most consistent with focal aortic dissection of uncertain acuity (Figure 2A,B). Given echo and CT scan findings, the patient was referred to our institution for surgical replacement of the ascending aorta and aortic root.
Operative technique
The femoral artery was exposed for cannulation and arterial inflow during cardiopulmonary bypass (CPB). All of the tissues were gossamer and extremely fragile. Median sternotomy was performed in a standard fashion. The aorta was quite sizable for that patient and enlarged progressively down towards the aortic root. Interestingly, on palpation behind the aortic root there were dense adhesions, consistent with severe irritation or chronic contained rupture. The bulging adventitia posteriorly was so thin that one hesitated to palpate for fear that just finger pressure would produce free rupture. Myocardial preservation was achieved by a combination of systemic hypothermia to 24 °C, topical hypothermia with iced saline, and cold crystalloid cardioplegia given retrograde through the coronary sinus. We cannulated the femoral artery (arterial inflow), right atrium (venous outflow), coronary sinus (retrograde cardioplegia), and right superior pulmonary vein (left heart vent).
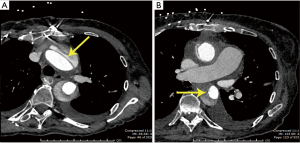
Aortotomy revealed an aortic root flap representing an old dissection in the non-coronary sinus with a markedly thinned, ping-pong ball size and shaped protrusion towards the left atrium from the non-coronary sinus (Figure 3). The patient had been very fortunate that this dissection had not propagated whenever it had occurred. Right and left coronary buttons were mobilized. Both of them had calcium in their perimeter which complicated their later reattachment. The peri-coronary button connective tissues were so fragile that most gave way just by picking up the strands even without application of electrocautery. We now prefabricated, on the back table, a biological valved conduit with a 21 Magna Ease valve (Edwards Lifesciences, Irvine, CA, USA) inside a 22 Hemashield graft (Maquet Cardiovascular, Rastatt, Germany) with running suture, which was then implanted into the annulus with interrupted pledgeted sutures.

The anastomosis of the left coronary button to an opening created in the back of the main graft was begun. At this point, we were informed by the perfusionist that the arterial pressure (left radial) had fallen to 20 mmHg. On palpation of the aorta, it was tense. We immediately turned our attention to the intraoperative transesophageal echocardiography (TEE), which showed an aortic dissection in the aortic arch and descending aorta extending up into the head vessels (Figure 4). We immediately packed the head in ice. The perfusionist was able to continue CPB flow at 2.6 L/min at 26 °C.

At this time, we expeditiously completed the left coronary button anastomosis and quickly finished the distal anastomosis to the aorta. We did these without the usual back wall reinforcements in order to speed the restoration of antegrade perfusion. After unclamping, we transferred the arterial cannula to the new graft. Diagnosis of the aortic dissection and completion of these two anastomoses and transfer of the arterial cannula with initiation of forward flow were all accomplished within 31 minutes.
With the institution of forward flow, the anesthesiologist was able to document, by TEE, flow into all the head vessels. When the dissection occurred, the gradient between the aortic root and the radial artery was 80 mmHg. After transferring the cannula, both pressures equalized at normal levels of 40–50 at full flow.
We then turned our attention to the right coronary button. We anastomosed this in a spatulated fashion to a beveled 8 mm Hemashield graft and then anastomosed that graft to the new ascending aortic graft. (We use an RCA interposition graft in 10% cases).
The heart had maintained an adequate rhythm and cardiac contraction even during the process of anastomosing the right coronary artery button. The patient was warmed to 34 °C and was weaned from CPB entirely without difficulty with vigorous myocardial function both in the field and by TEE. The overall cross-clamp time was 86 minutes. The total bypass time was 140 minutes. The patient was transferred to intensive care unit in stable condition, where anti-impulsive therapy was implemented and continued.
Postoperative course and follow-up
The early postoperative course was uneventful. The patient was alert and oriented with clear fluent speech and comprehension. Cranial nerves, motor and sensory functions were intact and reflexes symmetric. The TEE performed on the first postoperative day showed normal prosthetic valve function. A dissection of the descending aorta was visualized, with maximal aortic diameter of 3.46 cm. The aortic dissection was managed medically due to the lack of complications, such as rupture or impending rupture, organ ischemia, or rapid aortic dilatation. The patient was discharged home in stable condition 6 days after surgery. Six-weeks postoperative examination was normal and the patient felt excellent. Her neurological and cognitive functions were absolutely intact. The 6-week CT scan showed type B aortic dissection (Figure 5A,B).
In this case, continuing low flow perfusion at low temperature via the original cannula, rapidly completing the cross-clamp requiring work and reperfusion through the new graft led to excellent outcome from intraoperative aortic dissection. We saw little other option as direct cannulation at the moment of recognition of dissection would have required cannulating a dissected aortic arch or a dissected branch. After completion of the procedure, we followed our standard type B “complication specific” protocol.
We believe the very fragile aortic tissue rendered this patient liable to iatrogenic aortic dissection.
Comments
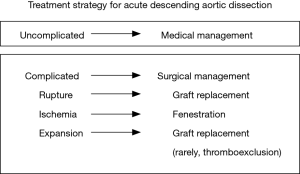
In the 1970s a group of clinicians from Stanford University determined that mortality was significantly improved in patients with acute ascending aortic dissection who were treated surgically (12). However, contemporaneously Dr. Appelbaum et al. found no clear benefit for surgical intervention in patients with stable acute aortic descending dissection (13). In 1992 our group at Yale published a series of descending dissection cases where patients were treated successfully by the “complication specific” approach (6). Patients who were free of rupture and ischemia from malperfusion were treated medically, while patients with the above-mentioned complications were treated surgically (Figure 6). This method is well-known and has been widely and successfully implemented in management of patients with IABD. Our recent study showed that a “complication specific” approach yielded 93% in-hospital survival and long-term survival of 83%, 78%, 71% and 47% at 1, 3, 5 and 10 years, respectively (9). Also, this study demonstrated that uncomplicated patients with IABD who were treated medically had the same long-term survival as a healthy control group (9).
Another question worth discussion regarding intra-operative aortic dissection has to do with the use of femoral cannulation for CBP. There are three most common sites for arterial cannulation: ascending aorta, axillary artery and femoral artery. Some studies state that axillary artery is the preferable site for cannulation for thoracic surgery because using the femoral artery incurs a higher risk for cerebral embolization initiated by retrograde flow (14,15). However, in our extensive published experience with femoral cannulation, patients have excellent survival, low stroke rates and minimum aortic rupture or dissections (16-18). Considering that axillary cannulation has its own complications (brachial plexus injury, arm ischemia, aortic dissection, etc.) (19,20), we use the femoral artery as our preferred site for perfusion in thoracic aortic interventions. We use axillary perfusion as our second choice when the descending/abdominal aorta is “dirty” by TEE or CT.
This case illustrates the successful management of IABD by continued low flow perfusion and postoperative “complication-specific” approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Still RJ, Hilgenberg AD, Akins CW, et al. Intraoperative aortic dissection. Ann Thorac Surg 1992;53:374-9; discussion 380. [Crossref] [PubMed]
- Williams ML, Sheng S, Gammie JS, et al. Aortic dissection as a complication of cardiac surgery: report from the Society of Thoracic Surgeons database. Ann Thorac Surg 2010;90:1812-6; discussion 1816-7.
- Hwang HY, Jeong DS, Kim KH, et al. Iatrogenic type A aortic dissection during cardiac surgery. Interact Cardiovasc Thorac Surg 2010;10:896-9. [Crossref] [PubMed]
- Januzzi JL, Sabatine MS, Eagle KA, et al. Iatrogenic aortic dissection. Am J Cardiol 2002;89:623-6. [Crossref] [PubMed]
- Fleck T, Ehrlich M, Czerny M, et al. Intraoperative iatrogenic type A aortic dissection and perioperative outcome. Interact Cardiovasc Thorac Surg 2006;5:11-4. [Crossref] [PubMed]
- Elefteriades JA, Hartleroad J, Gusberg RJ, et al. Long-term experience with descending aortic dissection: the complication-specific approach. Ann Thorac Surg 1992;53:11-20; discussion 20-1. [Crossref] [PubMed]
- Elefteriades JA, Lovoulos CJ, Coady MA, et al. Management of descending aortic dissection. Ann Thorac Surg 1999;67:2002-5; discussion 2014-9.
- Ziganshin BA, Dumfarth J, Elefteriades JA. Natural history of Type B aortic dissection: ten tips. Ann Cardiothorac Surg 2014;3:247-54. [PubMed]
- Charilaou P, Ziganshin BA, Peterss S, et al. Current Experience With Acute Type B Aortic Dissection: Validity of the Complication-Specific Approach in the Present Era. Ann Thorac Surg 2016;101:936-43. [Crossref] [PubMed]
- Gryaznov AA, Ma WG, Erben Y, et al. Aortic root flap representing an old dissection in the non-coronary sinus. Asvide 2018;5:524. Available online: http://www.asvide.com/article/view/24997
- Gryaznov AA, Ma WG, Erben Y, et al. Intraoperative TEE. Descending aortic dissection. Asvide 2018;5:525. Available online: http://www.asvide.com/article/view/24998
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Appelbaum A, Karp RB, Kirklin JW. Ascending vs descending aortic dissections. Ann Surg 1976;183:296-300. [Crossref] [PubMed]
- Battaloglu B, Erdil N, Nisanoglu V. Axillary artery perfusion in acute type A aortic dissection repair. J Card Surg 2008;23:693-6. [Crossref] [PubMed]
- Whitlark JD, Goldman SM, Sutter FP. Axillary artery cannulation in acute ascending aortic dissections. Ann Thorac Surg 2000;69:1127-8; discussion 1129. [Crossref] [PubMed]
- Ayyash B, Tranquilli M, Elefteriades JA. Femoral artery cannulation for thoracic aortic surgery: safe under transesophageal echocardiographic control. J Thorac Cardiovasc Surg 2011;142:1478-81. [Crossref] [PubMed]
- Fusco DS, Shaw RK, Tranquilli M, et al. Femoral cannulation is safe for type A dissection repair. Ann Thorac Surg 2004;78:1285-9; discussion 1285-9. [Crossref] [PubMed]
- Tsiouris A, Elkinany S, Ziganshin BA, et al. Open Seldinger-Guided Femoral Artery Cannulation Technique for Thoracic Aortic Surgery. Ann Thorac Surg 2016;101:2231-5. [Crossref] [PubMed]
- Apostolakis EE, Baikoussis NG, Katsanos K, et al. Postoperative peri-axillary seroma following axillary artery cannulation for surgical treatment of acute type A aortic dissection. J Cardiothorac Surg 2010;5:43. [Crossref] [PubMed]
- Schachner T, Nagiller J, Zimmer A, et al. Technical problems and complications of axillary artery cannulation. Eur J Cardiothorac Surg 2005;27:634-7. [Crossref] [PubMed]