Surgical management of cardiac implantable electronic device infections
Introduction
Cardiac implantable electronic devices (CIED), including permanent pacemakers (PPM), implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT) devices, have become important therapeutic tools with a continuously expanding field of application. The infection of these devices is a serious and potentially lethal complication. The population at risk of this type of infection is growing, as the frequency of device implantation is increasing, especially in older patients with associated comorbid conditions. This fact could explain the higher incidence of CIED infection observed in recent studies.
The management of these infections has been redefined, considering the high failure rates observed with antimicrobial treatment alone (1). Specifically, complete removal of every single component of the device (subcutaneous, intravenous and intracardiac) should be performed in most of the cases. These include not only patients with systemic infection, but also those with localized pocket infection (2).
Most studies describe specialized percutaneous methods for the removal (3,4). However, these procedures involve significant risks, including cardiac tamponade, pulmonary embolism and death, even in experienced hands. Thus, it is suggested that “only high-volume centers with appropriate facilities and training can perform these procedures relatively safely, with a high rate of success”.
The alternative to these methods, could be a classic open heart surgery approach. A cardiothoracic surgery team with sufficient experience in routine operations is capable for an effective device removal, with acceptable risks, provided that established cardiac surgical techniques are employed.
The aim of this study was to examine the clinical features, microbiological spectrum and echocardiographic findings in patients with established CIED infection referred to our Cardiothoracic Surgery department, in order to present the approach followed for the treatment of these patients and to evaluate the early and late outcomes observed.
Patients
During a 3-year period 1,508 CIED were implanted in our hospital. We treated six cases (64-84 years old) of permanent pacemaker infection between years 2008 and 2010 (2-10 years post implantation). The co-morbidities of these patients resulted in a high logistic Euro-score (6-35, 4%). They all presented with fever, and in four of them there was a pocket infection with skin erosion and purulent drainage. All patients were already under antibiotic treatment by the time they were transferred to our department. Duke criteria were checked for each patient, including pocket infection as a major criterion. Complete device removal was decided in all cases (endocarditis or localized pocket infection) in accordance to the recent AHA/ACC guidelines (2). All patients underwent preoperative coronary angiography, which in five patients resulted normal, and in one patient revealed a minor lesion, with no indication for aortocoronary bypass (Table 1).
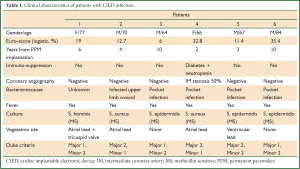
Full table
Methods
All patients were operated under general anaesthesia, with extracorporeal circulation. The infected pocket was isolated from the operative field. A midline sternotomy incision was made. The pericardium was opened; a ventricular bipolar temporary pacing wire was placed and connected with a temporary external pacemaker. Cannulation of the ascending aorta and inferior vena cava was performed; superior vena cava was excluded with a tight caval snare. Cardiopulmonary bypass went on, and an aortic vent was applied. Without aortic cross-clamping, the right atrium was opened on the beating heart; then both atrial and ventricular electrodes were pulled out as much as possible for about 5-8 cm from the superior vena cava and were transected. The cut distal ends were removed from the right heart chambers with gentle and firm traction. Debridement of reactive tissue around the atrial electrode site was made, when necessary. Electrodes and debridement tissue were sent for analysis as culture specimens. The right atriotomy was closed in a double running fashion with 4-0 prolene suture; each patient was rewarmed and weaned from the cardiopulmonary bypass. Decannulation was accomplished.
Epicardial permanent pacing electrodes were placed on the right atrium and ventricle. They were connected to the pacing generator and the latter was then implanted in a pocket created posterior to the rectus sheath. After the correct function of the permanent pacemaker was verified, the temporary pacing wire was removed. Haemostasis was secured from all sites and one mediastinal tube was placed. The sternotomy incision was then closed in layers.
Afterwards the old pacing generator was removed through a small incision over its pouch in the anterior chest wall, and the proximal parts of the electrodes were extracted by traction. In the two patients who did not present generator pocket infection the skin incision was closed. In the four patients with pocket infection the wound was left open to heal by second intention.
All patients tolerated the procedure well and were transferred to the ICU in stable condition.
Results
Staphylococcal species were responsible in all cases: two S. aureus, three S. epidermidis and one S. hominis. They were all methicillin-sensitive in the in vitro susceptibility testing. In four patients the transesophageal echocardiogram revealed vegetation on the atrial or ventricular lead, one of which was extended to the tricuspid valve.
The postoperative course of all patients was uncomplicated. They presented no febrile waves, and remained under antibiotic treatment (regimen that always included vancomycin or teicoplanin) for 40-57 days in total. The antibiotic treatment was always completed by intravenous administration before discharge. The patients were discharged in very good clinical condition and they were followed up till today (36-60 months) without any relapses (Table 2).

Full table
Discussion
Technologic advances in cardiac electrophysiology has led to an expansion of the devices implanted, from single to dual chamber PPM, followed by ICD and more recently by CRT devices. In an analysis of CIED implantation in the United States between 1997 and 2004, implantation rates for PPMs and ICDs increased by 19% and 60%, respectively (5).
Despite improvements in the design and implantation techniques, infection of cardiac devices remains a serious problem. The reported incidence of infection varies widely among studies, but seems to have been increased over the last years. In earlier years, the rates of PPM infection ranged between 0.13% (6) and 19.9% (7). A recent population-based study showed an incidence of CIED infection of 1.9 per 1,000 device-years and a higher probability of infection of ICDs as compared with PPMs (8). Data from another study point to a disturbing trend: infection rates are rising faster than implantation rates (9).
Staphylococcal species account for more than 2/3 of CIED infection cases in most published series, with Staphylococcus aureus and a variety of coagulase-negative Staphylococcus (CNS) species among them (2,10-12). CNS is well recognized as a common cause of microbiological specimen contamination, and this should be taken into account in the evaluation of culture results. Methicillin resistant staphylococci are more frequently isolated than methicillin sensitive ones, even if the exact prevalence of methicillin resistance among staphylococcal strains varies among studies. Gram-negative bacilli (including Pseudomonas aeruginosa), and Candida species account for a minority of CIED infections. Fungi other than Candida and nontuberculosis mycobacteria are rarely identified as pathogens in CIED infection (10,13-16).
The main mechanism of infection is the contamination of the generator pocket, either at the time of device implantation, or at a later time, following a cutaneous infection and/or erosion at the pocket site. Microorganisms from the pocket can spread along the electrode to the endocardium and the electrode tip. Less frequently the mechanism of infection is hematogenous seeding of the electrode during bacteremia of other origin.
The clinical presentation of a CIED infection may vary. Most commonly there is a local inflammatory reaction at the generator pocket area, or cutaneous erosion with exposure of the generator or the leads. These local signs are often accompanied by pain or discomfort, or vague symptoms like malaise, fatigue, anorexia, or decreased functional capacity. Fever and other signs of systemic inflammatory reaction are frequently, but not constantly, present. Less commonly, the diagnosis is suspected in patients with fever of undefined origin who present no local inflammatory signs at the generator pocket. Blood cultures, obtained before the beginning of any antibiotic treatment; represent an essential part of the diagnostic procedure, in all patients with CIED infection (2).
Local device infection and CIED-related infective endocarditis should be considered distinct clinical entities. Local device infection is defined as an infection limited to the generator pocket and is clinically suspected in the presence of local signs of inflammation, including erythema, warmth, fluctuance, wound dehiscence, erosion, tenderness, or purulent drainage. CIED-related infective endocarditis is confirmed when valvular or lead vegetations are detected by echocardiography, or if the Duke criteria for infective endocarditis are met (10). The advantage of transesophageal (TEE) over transthoracic (TTE) echocardiography in identifying pacemaker lead vegetations has already been demonstrated (12,17,18). In fact, performing a TEE exam is mandatory in all patients with suspected CIED-related endocarditis. By contrast, the use of positive lead cultures as a criterion for the diagnosis of endocarditis can be misleading, due to the high probability of contamination of the lead tip, during removal through an infected pocket. It has been proposed that positive lead cultures can be used as a sign of CIED-related infective endocarditis only in the absence of pocket infection or when the leads were removed using a remote incision from the pocket or by surgical extraction (17).
Earlier studies estimated that about 10% of all patients with CIED infections develop infective endocarditis (11,19,20). More recent studies report this incidence as high as 23-25% (17,21). However, the lower incidence of endocarditis between CIED infections in the earlier studies seems to be apparent and results in part, from the lack of a common definition of CIED-related endocarditis previously and to the more frequent use of echocardiography in the recent studies than in earlier reports.
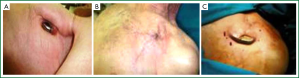
Management of a CIED infection must be based on the complete removal of the device (generator and leads), regardless of the extent of the infection, in combination with adequate antibiotic therapy (2,10,19,22). Infection of any part of a CIED implies contamination of the whole device. In fact, most studies have reported unacceptably high failure rates with conservative treatment (antibiotics and pocket debridement without device removal) (12,18,19,23-28) and high recurrence rates with lead-retention approach (extraction of the generator only) (29-32). An exception might be in our opinion the pocket erosion in a weak, scrawny patient (Figure 1) who presents with device or leads extraction due to chronic attrition. In these cases an alternative would be to reimplant the device, under local anesthesia, after careful rebuilding the pocket. We keep the device in Betadine solution 2% for five minutes and clean the pocket with the same solution and peroxide of hydrogen. At the end we place the device under the pectoralis major muscle or cover it subcutaneously with bovine pericardium. A vacuum aspiration in the pocket is placed for 24 hours. Although most patients present only with localized inflammatory signs at the generator pocket, this should not discourage physicians from removing the entire CIED, this being the only way to eradicate the infection.
Removal of an infected CIED can be performed either by percutaneous lead traction through the generator pocket, or by a surgical approach. Despite advances in techniques for percutaneous lead extraction, there are still considerable risks associated with this procedure. The most common complications reported include tearing of the tricuspid valve, damage to the myocardium, venous lacerations, bleeding with cardiac tamponade or hemothorax, pulmonary embolism, lead tip fracture resulting in incomplete removal, and pocket hematoma. Factors that increase the risk of these complications are the size of the vegetation (if present), the time from implantation, and the total number of leads. Large vegetations are more susceptible to fragmentation and septic embolism. Old leads are embedded in dense fibrous tissue, and consequently their removal by direct traction carries a higher risk of bleeding, myocardial perforation, valve tearing and venous laceration. The increased risk of these complications, when multiple leads are extracted, is obvious (10,12,24,33-36).
New techniques have been developed in order to minimize the above mentioned risks of percutaneous extraction, but their success depends on the availability of appropriate equipment (locking stylets, different types of sheaths, laser-powered tools) and on adequate training and experience. The high success rate and low complication rate reported by high-volume, specialized centers cannot be expected by centers with less operator experience or with smaller procedural volume (34,37). The surgical alternatives to percutaneous lead extraction have not yet received clear recommendations, mainly because of the various results reported by different studies, but also because surgery was usually compared with percutaneous extraction done in specialized centers with their obvious advantages. Surgical extraction is most commonly suggested when lead vegetations >1 or 2 cm are present, when severe tricuspid valve endocarditis is associated, when percutaneous extraction has been technically impossible or incomplete, or in cases of concomitant cardiac disease requiring surgical treatment. In cases of leads >12 months old, in which percutaneous extraction can be considered safe only in specialized centers using appropriate equipment, surgical extraction is the only alternative in centers non specialized, but with cardiothoracic surgery available. In any case, the good exposure of cardiac cavities, that open heart surgery offers, permits their direct exploration. Consequently, all manipulations on the leads are totally controlled. Another important advantage of surgical extraction is the possibility of immediate permanent epicardial pacemaker leads implantation. The new generator can be easily implanted behind the rectus sheath, an area that is easily accessible from the sternotomy incision, without the need of additional dissection (which would be the case if the device is implanted in the right subclavian area). Conversely, new transvenous leads should be implanted not earlier than a few days or weeks after percutaneous extraction. Immediate implantation of either temporary or permanent transvenous leads is not recommended, because it has been shown to be a risk factor for new CIED infection (10,38).
Complication rates of either percutaneous or surgical methods reported vary (10,24,39). Analysis of data from a registry of percutaneous lead extractions revealed a major complication rate of 1.6%. The four predictors of major complications were: (I) implant duration of oldest lead; (II) female gender; (III) ICD lead removal and (IV) use of laser extraction technique (40). Among 498 cases treated with percutaneous extraction in a specialized center, the total complication rate was 1%, of which 0.4% were major complications (37).
Evaluation of the complication rates of surgical extraction reported in recent studies should take into account the number of cases studied, the unhomogeneity of the surgical techniques used and the characteristics of the patients assigned to the surgical extraction group.
In a 10-year prospective study five cases of CIED infection (24) were assigned to the surgical treatment group. The inclusion criteria were long term leads (>12 months from implantation), large vegetations with higher embolic risk and cases with more than two leads. Two of these patients (40%) experienced major complications (SVC laceration and SVC massive thrombosis) and died. In a 13-year retrospective study, 19 patients with CIED infection (10) underwent lead removal by cardiotomy. Five of them (26%) suffered from serious complications: two massive haemorrhages (lethal in one patient, 5%), one cardiac arrest, one subclavian vein laceration, one ventriculotomy requiring operative repair. In a 10-year retrospective study 21 patients underwent open surgical lead extraction (41). Seven of them (33%) experienced complications, lethal in three patients (14%). The non lethal complications were hemothorax in two cases, pneumothorax in one case, and acute renal failure and deep sternal wound infection in one case.
In all cases of CIED infection that were referred to our cardiothoracic surgery department for further treatment, complete removal of the device was suggested. Removal by external manual traction could not be performed in our cases, mainly because of the risk of dissemination of the infection. A permanent epicardial pacemaker was implanted in all cases. All patients tolerated the procedure well and the postoperative course was uneventful without the aforementioned surgical complications. Also it is noteworthy that there is no relapse of the infection during the follow up period of five years.
It should be noted that our practice is to perform the operation with the aid of transesophageal echocardiogram, which provides detailed information about the size and length of vegetations, usually in the form of sleeves around the electrodes. We pull out the electrodes until we see a clean area and then we cut them with heavy scissors. Of great importance also, is the isolation of the infected pocket from the operative field of sternotomy.
Conclusions
The management of CIED infections remains a challenge, not only because of their increasing incidence and significant morbidity and mortality, but also considering that optimal care is not well defined.
Management protocols that include complete device removal are the only proven effective in the eradication of CIED infections. As newer technologies have emerged and experience has grown, highly specialized techniques of percutaneous device removal have been developed. The encouraging results of these techniques observed have reduced the frequency of serious complications. The surgical alternative to these methods can be a safe solution provided that simple, established principles of cardiac surgery are applied.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace 2010;12:64-70. [PubMed]
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458-77. [PubMed]
- Farooqi FM, Talsania S, Hamid S, et al. Extraction of cardiac rhythm devices: indications, techniques and outcomes for the removal of pacemaker and defibrillator leads. Int J Clin Pract 2010;64:1140-7. [PubMed]
- Jones SO 4th, Eckart RE, Albert CM, et al. Large, single-center, single-operator experience with transvenous lead extraction: outcomes and changing indications. Heart Rhythm 2008;5:520-5. [PubMed]
- Zhan C, Baine WB, Sedrakyan A, et al. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008;23 Suppl 1:13-9. [PubMed]
- Conklin EF, Giannelli S Jr, Nealon TF Jr. Four hundred consecutive patients with permanent transvenous pacemakers. J Thorac Cardiovasc Surg 1975;69:1-7. [PubMed]
- Bluhm G. Pacemaker infections. A clinical study with special reference to prophylactic use of some isoxazolyl penicillins. Acta Med Scand Suppl 1985;699:1-62. [PubMed]
- Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007;167:669-75. [PubMed]
- Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006;48:590-1. [PubMed]
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007;49:1851-9. [PubMed]
- Arber N, Pras E, Copperman Y, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore) 1994;73:299-305. [PubMed]
- Klug D, Lacroix D, Savoye C, et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation 1997;95:2098-107. [PubMed]
- Chacko ST, Chandy ST, Abraham OC, et al. Pacemaker endocarditis caused by Pseudomonas aeruginosa treated successfully. J Assoc Physicians India 2003;51:1021-2. [PubMed]
- Kouvousis N, Lazaros AG, Christoforatou EG, et al. Acremonium species pacemaker site infection. Hellenic J Cardiol 2003;44:83-7.
- Amin M, Gross J, Andrews C, et al. Pacemaker infection with Mycobacterium avium complex. Pacing Clin Electrophysiol 1991;14:152-4. [PubMed]
- Giannella M, Valerio M, Franco JA, et al. Pacemaker infection due to Mycobacterium fortuitum: the role of universal 16S rRNA gene PCR and sequencing. Diagn Microbiol Infect Dis 2007;57:337-9. [PubMed]
- Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008;83:46-53. [PubMed]
- Victor F, De Place C, Camus C, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart 1999;81:82-7. [PubMed]
- Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000;133:604-8. [PubMed]
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation 2003;108:2015-31. [PubMed]
- Villamil Cajoto I, Rodríguez Framil M, Van den Eynde Collado A, et al. Permanent transvenous pacemaker infections: An analysis of 59 cases. Eur J Intern Med 2007;18:484-8. [PubMed]
- Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin Electrophysiol 2000;23:544-51. [PubMed]
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004;350:1422-9. [PubMed]
- del Río A, Anguera I, Miró JM, et al. Surgical treatment of pacemaker and defibrillator lead endocarditis: the impact of electrode lead extraction on outcome. Chest 2003;124:1451-9. [PubMed]
- Bracke F, Meijer A, Van Gelder B. Extraction of pacemaker and implantable cardioverter defibrillator leads: patient and lead characteristics in relation to the requirement of extraction tools. Pacing Clin Electrophysiol 2002;25:1037-40. [PubMed]
- O'Nunain S, Perez I, Roelke M, et al. The treatment of patients with infected implantable cardioverter-defibrillator systems. J Thorac Cardiovasc Surg 1997;113:121-9. [PubMed]
- Molina JE. Undertreatment and overtreatment of patients with infected antiarrhythmic implantable devices. Ann Thorac Surg 1997;63:504-9. [PubMed]
- Rundström H, Kennergren C, Andersson R, et al. Pacemaker endocarditis during 18 years in Göteborg. Scand J Infect Dis 2004;36:674-9. [PubMed]
- Field ME, Jones SO, Epstein LM. How to select patients for lead extraction. Heart Rhythm 2007;4:978-85. [PubMed]
- Klug D, Wallet F, Lacroix D, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart 2004;90:882-6. [PubMed]
- Parry G, Goudevenos J, Jameson S, et al. Complications associated with retained pacemaker leads. Pacing Clin Electrophysiol 1991;14:1251-7. [PubMed]
- Wilhelm MJ, Schmid C, Hammel D, et al. Cardiac pacemaker infection: surgical management with and without extracorporeal circulation. Ann Thorac Surg 1997;64:1707-12. [PubMed]
- Meier-Ewert HK, Gray ME, John RM. Endocardial pacemaker or defibrillator leads with infected vegetations: a single-center experience and consequences of transvenous extraction. Am Heart J 2003;146:339-44. [PubMed]
- Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol 2008;31:736-52. [PubMed]
- Byrd CL, Wilkoff BL, Love CJ, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994-1996. U.S. Extraction Database, MED Institute. Pacing Clin Electrophysiol 1999;22:1348-57. [PubMed]
- Bracke FA, Meijer A, van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart 2001;85:254-9. [PubMed]
- Jones SO 4th, Eckart RE, Albert CM, et al. Large, single-center, single-operator experience with transvenous lead extraction: outcomes and changing indications. Heart Rhythm 2008;5:520-5. [PubMed]
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004;350:1422-9. [PubMed]
- Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009;6:1085-104. [PubMed]
- Wilkoff BL, Byrd CL, Love CJ, et al. Trends in intravascular lead extraction: analysis of data from 5339 procedures in 10 years. XIth World Symposium on Cardiac Pacing and Electrophysiology: Berlin Pacing Clin Electrophysiol 1999;22:A207.
- Camboni D, Wollmann CG, Löher A, et al. Explantation of implantable defibrillator leads using open heart surgery or percutaneous techniques. Ann Thorac Surg 2008;85:50-5. [PubMed]


