Impact of the favorable prognosis of patients with lung cancer adjoining bullae
Background
Lung cancer arising from bullae was first reported in 1941 (1), and is an uncommon manifestation. The detection of lung cancer associated with emphysematous bullae and cystic airspace increased because the use of thin slice computed tomography (CT) became widespread (2,3). Lung cancer associated with bullae has been observed in 3.4–13.3% (2,4). In addition, emphysematous bullae are important risk factors for lung cancer (5,6). The risk of lung cancer was 32 times higher in patients with bullae than in patients without bullae (6). Lung cancer adjoining bullae (LC-AB) is more susceptible to the carcinogenicity of cigarette smoking than lung cancer without bullae (4). However, the mechanism of carcinogenesis from bullae and its prognosis remains unclear.
The definition of airspaces in the lung is equivocal; for example, they are called bullae, blebs, cysts, and cystic airspaces. It is impossible to determine whether airspace is congenital or acquired (7). Moreover, it is unclear whether a tumor originated from the airspace, or developed adjacent to the airspace and then invaded (8). In this study, we defined bullae as emphysematous airspaces surrounded by lung parenchyma or visceral pleura with a diameter of more than 10 mm by evaluating radiological findings (9). Lung cancer associated with bullae is classified into 2 categories; LC-AB and apart from bullae (4). We focus on the former with radiographic findings and pathological manifestations, and excluded the latter in the present study.
Although LC-AB is well-recognized among radiologists (2), there are still many unclear points regarding its biological behavior and clinical characteristics, especially with regard to the prognosis. The purpose of this study is to investigate the clinical characteristics and the prognosis of patients with LC-AB.
Methods
Patients
We retrospectively reviewed 306 consecutive patients who underwent lung resection for lung cancer at Chubu Rosai Hospital (in Nagoya, Japan) between April 2007 and March 2015. Fifteen patients with incomplete resection were excluded.
We firstly detected LC-AB using radiography, as described in previous reports (4,10). LC-AB was then determined using both radiographic findings and pathological manifestations. The strict criteria for judging LC-AB is described in the definition of LC-AB section. LC-AB was observed in 52 patients (52/291, 17.9%).
The clinical features assessed were age, sex, comorbidities, smoking history, smoking index (pack-year), forced expiratory volume in 1 second per forced vital capacity (FEV1/FVC: FEV 1%), surgical procedure, location of tumor, invasive tumor size, pathological tumor-node-metastasis (TNM) classification [8th edition of the International Association for the Study of Lung Cancer Staging System (11)], and pathological findings. Follow-up was performed in our hospital and we checked the clinical outpatient records.
All surgeries were performed under the supervision of a thoracic surgeon. We performed lobectomy for lung cancer if patients could tolerate the procedure. We performed limited surgery as wedge resection or segmentectomy if the patients could not tolerate lobectomy. We performed lung resection through an anterior axillary 5–20 cm fourth or fifth intercostal thoracotomy incision or a posterior lateral 10–20 cm fourth intercostal thoracotomy incision.
This retrospective study was approved by the ethics committee of Chubu Rosai Hospital (number 201705-01). Because this study was retrospective, the requirement for informed consent was waived.
Definition of LC-AB
We judged LC-AB based on criteria for both radiographic and pathological findings.
- Bullae were defined as emphysematous airspaces with a diameter of 10 mm or more on thin slice CT;
- The circumferential portion of the lung nodule adjoining the bullae was more than 30° on thin slice CT imaging, as described in a previous report (2);
- The diameter of the thickened wall surrounding the bullae was less than 10 mm on thin slice CT imaging;
- Lung cancer with cavitary lesions containing fluid was excluded using radiographic findings;
- Cavities with necrotic tissue were excluded based on pathological findings.
The CT imaging findings were evaluated by two thoracic surgeons (S. Shinohara and M. Sugaya) individually and independently. The coincidence of both of the two judgements was needed to be recognized as LC-AB. A specialized pathologist (K. Kato) reviewed pathological slides to diagnose and evaluate airspace in all cases.
Evaluation of CT imaging
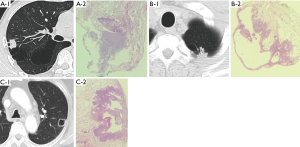
For all patients, CT was used to examine the brain, neck, chest, and abdomen within 1 month before surgery with a fixed lung window (width, 2,000; level, −500). We use Fuji Medical Systems for CT evaluation (Fuji Medical, Tokyo, Japan). The slice thickness was 1 to 5 mm. The following parameters were recorded: (I) tumor location (peripheral or central), (II) maximum diameter of the bullae, (III) thickness of the wall of the bullae, (IV) circumferential portion of the lung nodule adjoining the bullae (°), and (V) the association between the tumor and airspaces [endophytic nodule, exophytic nodule, or nodule surrounding the whole wall of the bullae (Figure 1)].
Pathological manifestations
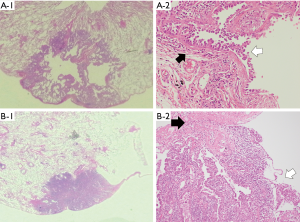
We evaluated the histological type (adenocarcinoma, squamous cell carcinoma, and others, including small cell lung cancer, neuroendocrine tumors, large cell carcinoma, pleomorphic carcinoma, and adenosquamous cell carcinoma), histological grade, vascular invasion, lymphatic invasion, pleural invasion, necrosis in airspaces, and the presence of bullae or emphysematous changes. We performed Elastica van Gieson staining to evaluate pleural, lymphatic, and vascular invasion. We divided the patients into 2 groups according to the formation of the tumor and bullae. The groups were (A) cancer cells lining the wall of the bullae (Figure 2A), and (B) cancer cells adjacent to the bullae (Figure 2B).
Statistical analysis
Data were expressed as the range, median, or mean, as appropriate. Comparative evaluation was performed using the Student’s t-test or Mann-Whitney U test for continuous variables and Fisher’s exact test or the χ2 test for categorical variables. Survival analysis was conducted using the Kaplan-Meier method and the estimates were compared using the log-rank test. We used a Cox proportional hazards model in univariate and multivariate analysis to identify prognostic factors. Baseline variables in univariate analysis with P<0.10 were added to the multivariate analysis. Overall survival (OS) was calculated from the date of surgery to the date of death from any cause or last contact and follow-up. Recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence or death. Patients who were lost to follow-up were censored at the date of the last follow-up. Statistical significance was determined at a P value of less than 0.05. All statistical analyses were performed using the R commander (R 3.4.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 291 patients with completely resected lung cancer were included in the analysis. At the time of completion of this research, 217 patients (74.6%) were alive and 74 had died (25.4%). The median follow-up length for all cases was 54.5 months [interquartile range (IQR), 34.0–75.7]. The percent of patients lost to follow-up was 6.2% (18/291).
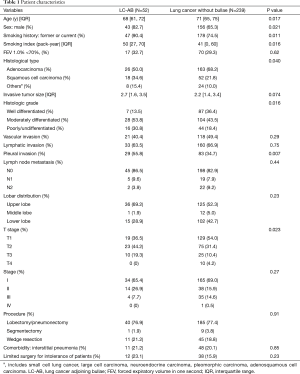
A total of 52 patients with LC-AB were identified. The clinical characteristics of all patients were summarized in Table 1. In the LC-AB group, the frequency of former or current smokers and male sex were significantly higher than in the non-LC-AB group (P=0.011 and 0.021, respectively). Age and smoking index were significantly higher in the LC-AB group (P=0.017 and 0.016, respectively). The tumor was located in the upper lobe in the LC-AB group more frequently than in the non-LC-AB group [36/52 (69.2%) vs. 125/239 (52.3%), P=0.031]. Pathological assessment revealed that the LC-AB group had a higher frequency of pleural invasion (P=0.007) and histological type of non-adenocarcinoma [26/52 (50.0%) vs. 76/239 (31.8%), P=0.016]. Furthermore, the histological grade was more frequently moderately or poorly differentiated in the LC-AB group than the non-LC-AB group [44/52 (84.6%) vs. 148/239 (61.9%), P<0.001].
Full table
Profile of LC-AB
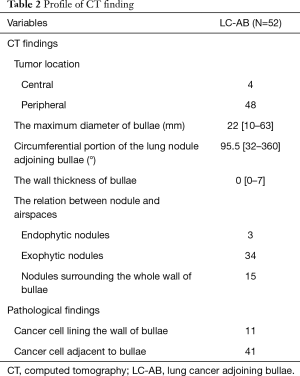
We described the radiographic findings and pathological manifestations of LC-AB in Table 2. Ninety-two percent of LC-AB was located in the periphery (48/52). The median maximum diameter of the bullae was 22 (range, 10–63) mm. The median wall thickness was 0 (range, 0–7) mm. The median circumferential portion of the lung nodule adjoining bullae was 95.5° (range, 32°–360°). There were 3 endophytic nodules, 34 exophytic nodules, and 15 nodules surrounding the whole wall of the bullae.
Full table
In pathological findings, there were 11 cases in which the cancer cells lined the wall of the bullae, and 41 cases in which the cancer cells were adjacent to the bullae.
Survival analysis
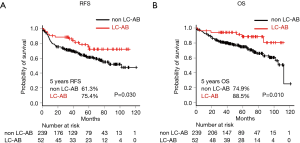
Kaplan-Meier analysis showed that RFS was significantly higher in LC-AB than non-LC-AB (P=0.030, Figure 3A) with a 5-year RFS rate of 75.4% and 61.3%, respectively. OS was significantly higher in the LC-AB group than the non-LC-AB group (P=0.010, Figure 3B) with 5-year survival rates of 88.5% and 74.9%, respectively.
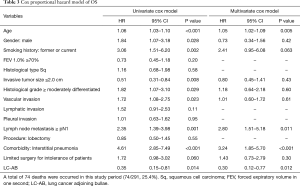
Univariate analysis for the Cox proportional hazard model of OS was summarized in Table 3. The probability of OS was associated with age [hazard ratio (HR): 1.06, 95% confidence interval (CI): 1.03–1.10, P<0.001], male sex (HR: 1.84, 95% CI: 1.07–3.18, P=0.028), former or current smoking (HR: 3.06, 95% CI: 1.51–6.20, P=0.002), invasive tumor size ≤2.0 cm (HR: 0.51, 95% CI: 0.31–0.84, P=0.008), histological grade ≥ moderately differentiated (HR: 1.82, 95% CI: 1.07–3.10, P=0.029), vascular invasion (HR: 1.72, 95% CI: 1.08–2.75, P=0.023), lymph node metastasis ≥ pN1 (HR: 2.35, 95% CI: 1.39–3.98, P=0.001), interstitial pneumonia (HR: 4.61, 95% CI: 2.85–7.49, P<0.001), and LC-AB (HR: 0.35, 95% CI: 0.15–0.81, P=0.014).
Full table
Multivariate analysis for the Cox proportional hazard model of OS is shown in Table 3. Independent factors associated with OS were age (HR: 1.05, 95% CI: 1.02–1.09, P=0.005), lymph node metastasis ≥ pN1 (HR: 2.80, 95% CI: 1.51–5.18, P=0.011), interstitial pneumonia (HR: 3.24, 95% CI: 1.85–5.70, P<0.001), and LC-AB (HR: 0.30, 95% CI: 0.12–0.77, P=0.012).
Discussion
In this study, we investigated the characteristics and prognosis of patients with LC-AB. The LC-AB group demonstrated a higher frequency of former or current smokers, male sex, tumor location in the upper lobe, histological type of non-adenocarcinoma, moderately or poorly differentiated histological grade, and pleural indentation. The LC-AB group showed significantly higher OS and RFS. Multivariate analysis for the Cox proportional hazard model of OS showed that LC-AB is an independent favorable prognostic factor.
LC-AB is an uncommon manifestation, making it difficult to define and determine appropriate diagnostic criteria. Although we referred to previous reports (4,12) to define LC-AB, there were no obvious definitions to exclude cavitary lesions and no pathological approaches. Therefore, we developed criteria to exclude cavitary lesions using both radiographic and pathological manifestations. Moreover, we determined lung nodule adjoining bullae when the circumferential portion of the lung nodule adjoining bullae was more than 30°. A long-term observational study for lung cancer-associated cystic airspaces reported that the median circumferential portion of the lung nodule adjoining bullae was 90° (range, 60°–360°) (2), which is similar to that reported in our study; therefore, our criteria seem to be feasible.
The clinical characteristics of LC-AB showed that LC-AB was more likely to be observed in men, smokers (12,13), and young patients (4). The present study showed that the upper lobe was more susceptible to LC-AB. These characteristics suggested that LC-AB is closely associated with cigarette smoking because cigarette smoking mainly causes emphysematous changes in the upper lobe. There are 2 possible reasons why emphysematous changes and bullae increase the incidence of carcinogenesis. First, patients with LC-AB may be sensitive to DNA damage caused by cigarette smoking (4). Second, chronic inflammation in bullae promotes carcinogenesis and delays the recovery from DNA damage (13,14).
The malignant manifestation in histology of LC-AB was higher than that of non-LC-AB. Pleural invasion was more frequent (12) and the histological differentiation was lower than that of non-LC-AB (12,13). The results of these previous reports are consistent with those of the present study. The malignant potential of LC-AB may be higher than that of non-LC-AB because of accumulated DNA damage caused by cigarette smoking (4), a higher frequency of programmed death-ligand 1 expression in LC-AB (12), and activated matrix metalloproteinases in the emphysematous lung (15). In fact, Kimura et al. reported the worse OS of LC-AB than the control group (37.0% vs. 91.7%, P<0.001), although the number of LC-AB was only 12 cases (16).
Despite the apparent higher malignant potential in pathological manifestation of LC-AB, the present study showed that 5-year survival of the LC-AB group was significantly better than that of the non-LC-AB group. This result with the better survival in LC-AB group although with the higher malignant pathological finding seems to be inconsistent. However, the better prognosis of LC-AB found in our study could be explained by the following 4 reasons. First, lung cancer associated with chronic obstructive pulmonary disease (COPD) may be less malignant. A single institutional retrospective study showed that COPD-related lung cancer presented with lower aggressiveness in its molecular and biological features; there were an increased lepidic component, a reduced solid pattern, a lower Ki-67/MIB1 index, and less frequent KRAS mutations compared with lung cancer without COPD (17). Second, a previous report showed the 5-year survival rate in LC-AB was not lower than in non-LC-AB, although there was poorer differentiation and higher proliferative activity in LC-AB (13). Because bullae are simple airspaces and do not contain any cell components, the bullae walls can protect the development of cancer cells. Third, we excluded cavitary lung cancer, which is one of the worst prognostic factors (18,19). We defined and excluded cavitary lung cancer when the airspaces had a thickened wall of less than 10 mm. Considering a previous report that indicated that a cavitary wall thickness of greater than 4 mm was an independent worse prognostic factor (19), our series of LC-AB may be less invasive because the median wall thickness was 0 mm (range, 0–7 mm). Fourth, pulmonary function after major lung resection in COPD patients was preserved because of a volume reduction effect, with the present series including large number of cases of upper lobe lung cancer. Upper lobectomy can improve pulmonary function in COPD patients (20,21); therefore, in the present study, patients with LC-AB had a favorable prognosis.
We acknowledge that our study has some limitations. First, the judgement of LC-AB is influenced by individual subjectivity; nevertheless, we tried to minimize discrepancies using strict criteria for the determination of LC-AB. Second, recall bias and selection bias is unavoidable because this is a retrospective, single institution study. Third, the heterogeneity of the present study may mislead to incorrect analysis because the present study included patients underwent pneumonectomy, lobectomy, segmentectomy, and wedge resection. We should focus on only lobectomy, although the number of patients was insufficient.
In conclusion, we found that patients with LC-AB had higher OS and RFS than those with non-LC-AB. Moreover, LC-AB was an independent favorable prognostic factor in multivariate analysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the ethics committee of Chubu Rosai Hospital (number 201705-01). Because this study was retrospective, the requirement for informed consent was waived.
References
- Womack NA, Graham EA. Epithelial metaplasia in congenital cystic disease of the lung: Its possible relation to carcinoma of the bronchus. Am J Pathol 1941;17:645-54.5.
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Tsutsui M, Araki Y, Shirakusa T, et al. Characteristic radiographic features of pulmonary carcinoma associated with large bulla. Ann Thorac Surg 1988;46:679-83. [Crossref] [PubMed]
- Iwama E, Okamoto I, Yabuuchi H, et al. Characteristics of Smoking Patients with Lung Cancer with Emphysematous Bullae. J Thorac Oncol 2016;11:1586-90. [Crossref] [PubMed]
- KOROL E. The correlation of carcinoma and congenital cystic emphysema of the lungs; report of ten cases. Dis Chest 1953;23:403-11. [Crossref] [PubMed]
- Stoloff IL, Kanofsky P, Magilner L. The risk of lung cancer in males with bullous disease of the lung. Arch Environ Health 1971;22:163-7. [Crossref] [PubMed]
- Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr 2015;39:102-8. [Crossref] [PubMed]
- Fintelmann FJ, Brinkmann JK, Jeck WR, et al. Lung Cancers Associated With Cystic Airspaces: Natural History, Pathologic Correlation, and Mutational Analysis. J Thorac Imaging 2017;32:176-88. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Nickoladze GD. Bullae and lung cancer. J Thorac Cardiovasc Surg 1993;106:186. [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Toyokawa G, Takada K, Okamoto T, et al. High Frequency of Programmed Death-ligand 1 Expression in Emphysematous Bullae-associated Lung Adenocarcinomas. Clin Lung Cancer 2017;18:504-11.e1. [Crossref] [PubMed]
- Hanaoka N, Tanaka F, Otake Y, et al. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer 2002;38:185-91. [Crossref] [PubMed]
- Goldstein MJ, Snider GL, Liberson M, et al. Bronchogenic carcinoma and giant bullous disease. Am Rev Respir Dis 1968;97:1062-70. [PubMed]
- Finlay GA, O’Driscoll LR, Russell KJ, et al. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med 1997;156:240-7. [Crossref] [PubMed]
- Kimura H, Saji H, Miyazawa T, et al. Worse survival after curative resection in patients with pathological stage I non-small cell lung cancer adjoining pulmonary cavity formation. J Thorac Dis 2017;9:3038-44. [Crossref] [PubMed]
- Schiavon M, Marulli G, Nannini N, et al. COPD-related adenocarcinoma presents low aggressiveness morphological and molecular features compared to smoker tumours. Lung Cancer 2014;86:311-7. [Crossref] [PubMed]
- Onn A, Choe DH, Herbst RS, et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology 2005;237:342-7. [Crossref] [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Cavity Wall Thickness in Solitary Cavitary Lung Adenocarcinomas Is a Prognostic Indicator. Ann Thorac Surg 2016;102:1863-71. [Crossref] [PubMed]
- Korst RJ, Ginsberg RJ, Ailawadi M, et al. Lobectomy improves ventilatory function in selected patients with severe COPD. Ann Thorac Surg 1998;66:898-902. [Crossref] [PubMed]
- Rapicetta C, Tenconi S, Voltolini L, et al. Impact of lobectomy for non-small-cell lung cancer on respiratory function in octogenarian patients with mild to moderate chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2011;39:555-9. [Crossref] [PubMed]


