Surgery for pulmonary metastases from colorectal cancer: survival and prognostic factors
Introduction
Evidence regarding pulmonary metastasectomy for colorectal cancer (CRC) consists almost exclusively of retrospective studies with variable results (1). Randomized controlled trials has yet to publish results to help inform and guide clinical practice, and high-quality evidence supporting surgery is lacking (2), but retrospective studies could help to identify prognostic factors that can aid in the selection of patients likely to benefit from surgery. Prognostic factors suggested by prior studies include staging of primary tumor, number and laterality of metastases, disease-free interval (DFI), thoracic lymph node status, previous metastatic disease in the liver, carbohydrate antigen 19-9, pre-thoracotomy carcinoembryonic antigen (CEA), K-RAS gene status and location of the primary tumor (3-9). However, no clear consensus has been reached as to how these factors should be used when recommending surgery.
A recent multicenter study from Japan proposed a prognostic index based on five preoperative variables: DFI <2 years, presence of extrathoracic lesions, abnormal CEA level, ≥3 pulmonary metastases, and age ≥70 years (8). The authors found that patients could be classified into three risk groups based on these prognostic factors and that overall survival was significantly different in each risk stratum (8). However, before a clinical risk prediction model can be applied clinically, evaluation of the performance in a population separate from the derivation data set (external validation) is necessary (10).
We obtained individual-level data from ThoR (Thoracic surgery Register), a Swedish national quality register for thoracic surgery, and performed a nationwide population-based observational cohort study in all patients who underwent pulmonary metastasectomy for CRC. The aims were to describe overall survival following pulmonary metastasectomy for CRC in Sweden, and to assess the discriminatory capacity of a recently proposed risk prediction model for survival by external validation.
Methods
The study was approved by the regional Human Research Ethics Committee, Stockholm, Sweden (Dnr: 2014/129-31/1 and 2015/2338-32). The need for informed consent was waived by the committee.
Study design
This observational population-based cohort study followed the STROBE and RECORD guidelines for observational studies using routinely collected data (11,12).
Patients and outcomes measures
We used ThoR (http://www.ucr.uu.se/thor), to identify the study population. We included all patients registered in ThoR who underwent surgical resection of pulmonary metastases from CRC between January 01, 2009 and December 31, 2015. ThoR is a quality register for general thoracic surgery, mainly procedures of the lungs and pleura. Although ThoR was started in 2008, a complete coverage of all eight thoracic surgery departments in Sweden was not achieved until 2013. From 2009 to 2011, approximately 50% of all patients who underwent thoracic surgery in Sweden were included, and during 2011 and 2012, seven out of eight hospitals reported to ThoR. The primary outcome measure was all-cause mortality. Vital status was determined on April 15, 2017, by using the Swedish personal identity number (13) and the continuously updated Swedish population register (14). Follow-up was 100% complete.
In general terms, the indications for surgery were in line with clinical practice guidelines (15). Under the following conditions, patients were recommended pulmonary metastasectomy: primary tumor was treated radically, no extra-pulmonary metastases except synchronous liver metastases that was deemed treatable radically, the pulmonary metastases were judged to be resectable, and the patient should be otherwise fit for major surgery. Treatment of the primary tumor and possible liver metastases was usually performed prior to pulmonary resection.
Definitions
Comorbidity was defined as any major medical condition that required ongoing treatment or could influence prognosis, e.g., heart disease, diabetes, or history of stroke. The extent of lung resection was divided into sublobar resection versus lobectomy. Smoking status was divided into four categories: current, former, never, and unknown. Current smoker was defined as an active smoker or a person who had stopped smoking within 1 month of surgery. Former smoker was defined as a previous smoker who had stopped smoking more than 1 month before surgery. Never smoker was defined as a person who had never been an active smoker.
Prognostic index and risk categories
The prognostic index was calculated as described by Okumura et al. (8) by the presence of five preoperative prognostic factors: age ≥70 years, DFI <2 years, existence of an extra-thoracic lesion, abnormal pre-thoracotomy CEA level, ≥3 pulmonary metastases. Patients were assigned to one of three risk categories based on the number of preoperative prognostic factors (0 factors: low risk, 1–2 factors: moderate risk, ≥3 factors: high risk).
Missing data
We used multiple imputation by chained equations (16) to handle missing data in 188 (25%) patients regarding the variable DFI. The imputation model included all variables reported in Table 1, year of surgery, hospital, event indicator, and the Nelson-Aalen estimator of the cumulative baseline hazard (17). Fifty data sets were imputed and estimates from these data sets were combined. We did not have information regarding preoperative CEA level for any of the patients in this study because ThoR does not collect this information. For the purpose of this study, all patients were considered to have normal CEA in the calculation of the prognostic index.
Full table
Statistical analyses
Baseline characteristics were described with frequencies and percentages for categorical variables and mean and standard deviation for continuous variables. Person-time in days was counted from the date of operation until the date of death or the end of follow-up (April 15, 2017). The Kaplan-Meier estimator was used to calculate cumulative survival in the total population and according to risk category. We estimated the restricted mean survival time (18) which is the average duration of survival over a given time period, in the three risk categories, and calculated the difference [95% confidence interval (CI)] in survival compared with the low-risk category. We used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for the association between risk category and all-cause mortality. Statistical analyses were performed using Stata version 15.1 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
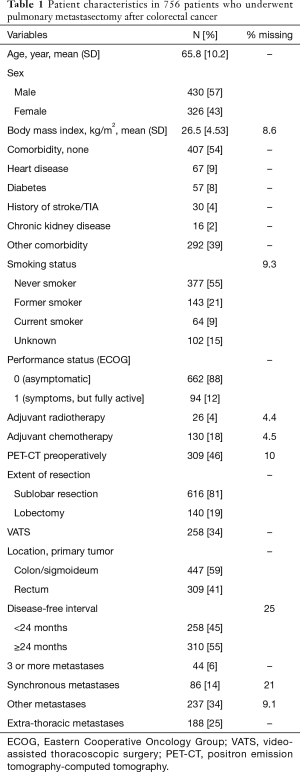
During the study period, 756 patients underwent 929 operations. For patients who had multiple entries in the register, only the first record was used, and a unique patient was therefore included in the study only once. Patient characteristics are shown in Table 1. The mean age was 65.8 years and 43% were women. The majority (88%) were free from symptoms (performance status 0), and 54% had no known comorbidity. Preoperative positron emission tomography-computed tomography (PET-CT) has been performed in 46% of the patients. The location of the primary CRC tumor was the colon or sigmoideum in 59% and the rectum in 41%. Three or more metastases were found in 6%, and 25% had extrathoracic metastases. The location of extrathoracic metastases was mainly the liver (90%), and in the remaining 10%, it was not specified. In 34%, the pulmonary resection was performed by video-assisted thoracoscopic surgery (VATS), and the majority (81%) had a sublobar resection.
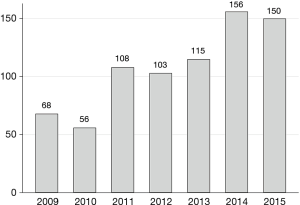
Number of patients operated per year
The number of operations per year is shown in Figure 1. The apparent increase in the number of patients operated likely reflects the fact that ThoR did not include all hospitals in Sweden performing thoracic surgery until 2013.
Survival in the total study population
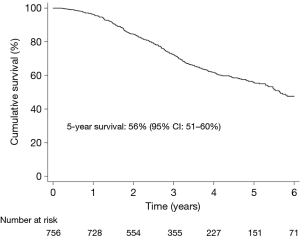
No patient died within 30 days of surgery. During a median follow-up time of 2.9 years, 35% (268/756) patients died. Figure 2 shows the Kaplan-Meier estimated overall survival in the total study population. At 5 years, overall survival was 56% (95% CI: 51–60%).
Survival according to risk category
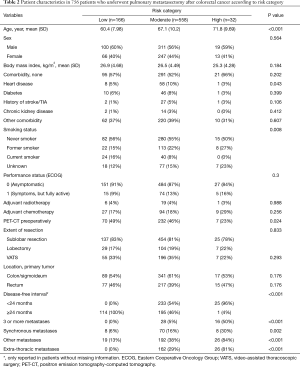
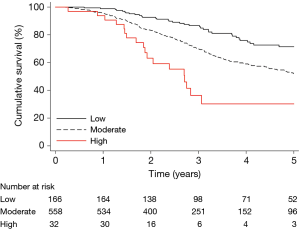
The patients were classified into three risk categories as described above: low (n=166), moderate (n=558), and high (n=32) risk. Of the preoperative prognostic factors that were used in combination to classify patients into risk categories, only number of metastases (≥3) was significantly associated with all-cause mortality in univariate analysis (HR: 2.47; 95% CI: 1.65–3.68, P<0.001). The baseline patient characteristics according to risk category are shown in Table 2, and the Kaplan-Meier estimated overall survival is shown in Figure 3. By visual assessment of the graph, the survival curves in the low and moderate categories were well separated at 1 year. After 2 years, the survival curves for all three risk categories were well separated. In a Cox regression model with risk category as the only independent variable, the HR was 1.94 (95% CI: 1.38–2.72, P<0.001) and 4.35 (95% CI: 2.49–7.62, P<0.001), in the moderate- and high-risk categories, respectively, compared with the low-risk category (C-statistic 0.58). The difference in restricted mean survival time at various follow-up times between the moderate- and high-risk categories, and the reference category (low-risk category) is shown in Table 3. At 2 years, the difference in restricted mean survival time was 1 month in the moderate-risk category versus the low-risk category (P<0.001), and 2 months in the high versus the low risk category (P<0.001). At 5 years, the difference in restricted mean survival time was 6 months in the moderate- versus the low-risk category (P<0.001), and 1.5 years in the high versus the low risk category (P<0.001).
Full table

Full table
We found significantly better survival in patients who underwent VATS compared to open procedures in an analysis adjusted for risk category (HR 0.73; 95% CI: 0.55–0.97, P=0.028). Moreover, we found no difference in survival in patients who underwent lobectomy compared to sublobar resection in an analysis adjusted for risk category (HR 1.29; 95% CI: 0.97–1.72, P=0.078).
Discussion
The 5-year survival after surgery for pulmonary metastases from CRC in Sweden was 56%, which is similar or higher in comparison with other contemporary reports in the literature. Our data suggest that the current selection criteria for pulmonary metastasectomy were sound. This is a particularly important finding because definitive guidelines for patient selection are lacking. In addition, we showed that a recently proposed prognostic model for survival that was derived in a Japanese patient population also had excellent discrimination in an external validation cohort of Swedish patients. Patients who were categorized as low risk had a statistically significant better survival compared with patients in the moderate- or high-risk groups. More importantly, the difference was clinically relevant and was quantified in absolute terms, which may be more informative to patients and clinicians alike.
CRC and pulmonary metastases
CRC is one of the most common cancer types in both sexes, with an estimated 1.4 million cases globally (19,20). During 1995–2009, more than 1.9 million cases were reported in North America alone (21). In Sweden, roughly 6,000 new cases of CRC are diagnosed annually (15). Approximately 14–19% will have metastatic growth at primary diagnosis, and this portion will increase to nearly 50% during follow-up (22-24). The most common organ with CRC metastatic disease is the liver, affecting about 35%, followed by the lungs, affecting about 15% of patients (6,25). Over time, the reported 5-year survival has increased from about 25% to more than 50% today (22-24). However, for patients with metastatic disease, 5-year survival is still only about 14% (23,24). This improving trend is likely attributable to multiple factors such as earlier diagnosis, preoperative radiotherapy, mesorectal excision, and reduction in postoperative mortality (21). In the case of metastatic disease in the liver, improved survival has been attributed to hepatic metastasectomy and improved chemotherapy (26). These optimistic results in patients with hepatic metastases have likely contributed toward more aggressive surgical treatment of pulmonary metastases, leading to growing numbers of pulmonary metastasectomy procedures (7,27). It has been speculated that some of the survival benefit might be attributable to other factors including selection bias, lead-time bias and staging migration (2). One randomized controlled trial (the Pulmonary Metastasectomy in Colorectal Cancer trial) (1,2) enrolled 93 patients in a feasibility study (https://clinicaltrials.gov/ct2/show/NCT01106261), to determine the possibility of conducting a sufficiently large randomized controlled trial to investigate the value of pulmonary metastasectomy in patients with CRC, but the results have not yet been published.
Prognostic factors for survival
The 5-year survival was 56%, which was similar or higher compared with previously reported overall survival following pulmonary metastasectomy in CRC (6). A systematic review and meta-analysis that included 25 studies reported that the 5-year survival ranged from 27% to 68% (6). Numerous studies have attempted to identify prognostic factors to guide and inform clinicians in the selection of patients who will most benefit from surgical treatment with pulmonary metastasectomy (3-9,28). Several factors useful for prediction of survival following pulmonary metastasectomy in CRC have been suggested: CEA level (4-8), DFI (5,6,8), number of pulmonary metastases (6,8), and thoracic lymph node involvement (5,6,9). A small number of studies have sought to combine these and other factors into multivariable risk prediction models to improve the discriminatory capacity, compared with the use of single prognostic factors (5,8,29). Thus far, the performance of these models has not been evaluated in populations separate from the patient populations they were derived from, a process known as external validation (10). This process involves assessment of the risk model performance, often by investigation of model discrimination and calibration. External validation is required before widespread use of the risk prediction model can be recommended (10).
Okumura et al. (8) recently proposed an easily applicable risk prediction model for survival following pulmonary metastasectomy in CRC by combining five prognostic factors and found clear discrimination among risk categories in a large population of Japanese patients. We applied a modified version of a risk prediction model proposed by Okumura et al. (8) in Swedish patients with CRC who had undergone pulmonary metastasectomy and found that the model successfully provided risk stratification in an external validation cohort. Our findings suggest that the risk prediction model proposed by Okumura et al. (8) could aid surgeons in their effort to provide the best care and quality of life for patients with pulmonary metastases after treatment of CRC.
Study limitations
This was an observational, retrospective study with data obtained from a national quality register. The most important limitation of the study was the lack of information regarding preoperative CEA levels, which was not included in ThoR. Consequently, some patients were incorrectly classified into the low- or moderate-risk categories instead of the moderate- or high-risk categories. Moreover, the study was restricted to the assessment of discrimination, which is only one aspect of the performance of a risk prediction model. A comprehensive external validation study should also include assessment of calibration (10), which was not possible in this case partly because of lack of information regarding CEA levels. Another limitation of our study was that the ThoR register did not reach a complete coverage of all eight thoracic surgery departments in Sweden until 2013, and that there was missing data regarding DFI.
Conclusions
Five-year survival following pulmonary metastasectomy in CRC in Sweden was satisfactory in comparison with previously published series, suggestive of adequate patient selection criteria. We also showed that a prognostic model for survival, initially developed in population of 785 Japanese patients, successfully provided risk stratification in an external validation cohort of Swedish patients.
Acknowledgements
The authors thank the ThoR steering committee for providing data for this study. Dr. Franco-Cereceda was supported by a donation from Mr. Fredrik Lundberg.
Funding: Dr. Sartipy was supported by grants from the Swedish Heart-Lung Foundation (grant numbers 20160522 and 20160525), the Mats Kleberg Foundation (2017-00096), Karolinska Institutet Foundations and Funds (2016fobi47721), Swedish Heart and Lung Association (E101/16), Åke Wiberg Foundation (M17-0089), Magnus Bergvall Foundation (2017-02054), and the regional ALF agreement between Stockholm County Council and Karolinska Institutet (20160329).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the regional Human Research Ethics Committee, Stockholm, Sweden (Dnr: 2014/129-31/1 and 2015/2338-32). The need for informed consent was waived by the committee.
References
- Treasure T, Fallowfield L, Lees B, et al. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. Thorax 2012;67:185-7. [Crossref] [PubMed]
- Åberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg 2016;50:792-8. [Crossref] [PubMed]
- Zampino MG, Maisonneuve P, Ravenda PS, et al. Lung metastases from colorectal cancer: analysis of prognostic factors in a single institution study. Ann Thorac Surg 2014;98:1238-45. [Crossref] [PubMed]
- Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435-40. [Crossref] [PubMed]
- Embun R, Rivas de Andres JJ, Call S, et al. Causal Model of Survival After Pulmonary Metastasectomy of Colorectal Cancer: A Nationwide Prospective Registry. Ann Thorac Surg 2016;101:1883-90. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Al-Ameri M, Persson M, Bergman P, et al. Long-term survival after surgery for pulmonary metastases from colorectal cancer: an observational cohort study. J Thorac Dis 2017;9:4358-65. [Crossref] [PubMed]
- Okumura T, Boku N, Hishida T, et al. Surgical Outcome and Prognostic Stratification for Pulmonary Metastasis From Colorectal Cancer. Ann Thorac Surg 2017;104:979-87. [Crossref] [PubMed]
- Cho JH, Hamaji M, Allen MS, et al. The prognosis of pulmonary metastasectomy depends on the location of the primary colorectal cancer. Ann Thorac Surg 2014;98:1231-7. [Crossref] [PubMed]
- Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12. [Crossref] [PubMed]
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659-67. [Crossref] [PubMed]
- Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125-36. [Crossref] [PubMed]
- Nationellt vårdprogram 2016. Tjock- och ändtarmscancer (Swedish). 2016. . Accessed Jan 11, 2018.http://www.cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/vardprogram/nvpkolorektalcancer_2016-03-15.pdf
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. [Crossref] [PubMed]
- White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982-98. [Crossref] [PubMed]
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol 2012;18:25-33. [Crossref] [PubMed]
- White A, Joseph D, Rim SH, et al. Colon cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:5014-36. [Crossref] [PubMed]
- Joseph DA, Johnson CJ, White A, et al. Rectal cancer survival in the United States by race and stage, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:5037-58. [Crossref] [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. [Crossref] [PubMed]
- Petrella F, Diotti C, Rimessi A, et al. Pulmonary metastasectomy: an overview. J Thorac Dis 2017;9:S1291-8. [Crossref] [PubMed]
- Dudek W, Schreiner W, Hohenberger W, et al. Forty-Two Years' Experience with Pulmonary Resections of Metastases from Colorectal Cancer. Thorac Cardiovasc Surg 2017;65:560-6. [Crossref] [PubMed]
- Salah S, Watanabe K, Welter S, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol 2012;23:2649-55. [Crossref] [PubMed]