Free musculocutaneous flap transfer for refractory chronic empyema with chest wall sinus in a 43-year-old male with hemophilia A
Introduction
Chronic empyema combined with chest wall sinus is a refractory and agonizing disease resulting from multiple predisposing etiologies. Successful management remains challenging. Hemophilia A is a sex-linked recessive hereditary disease in which the blood does not clot normally due to lack of coagulation factor VIII and may lead to high risk of excessive surgical bleeding. There have been a few reports on major surgery but no report on free musculocutaneous flap transfer for chronic empyema with chest wall sinus in patients with hemophilia A (1,2). It is crucial not only to completely obliterate infective diseased space but also minimize the risk of surgical bleeding perioperatively. We herein report a case of a free vastus lateralis musculocutaneous flap transfer for chronic empyema combined with chest wall sinus in a 43-year-old male with hemophilia A under the administration of recombinant coagulation factor VIII without any hemorrhagic complication.
Case presentation
A 43-year-old male with a history of hemophilia A developed left chronic empyema combined with chest wall sinus for 4 months. For 19 years ago, he presented to emergency department with moderate thoracic trauma by car accident. Because hemothorax was increased gradually under observation, he then underwent urgent thoracotomy. No bleeding spot was found after removal of bloody effusion and hemostasis without lung resection was performed. Due to excessive bleeding during and after surgery, he was suspicious of coagulation disorders. One week later, he was definitely diagnosed with hemophilia A by deficiency of coagulation factor VIII, whose activity was only 10%.
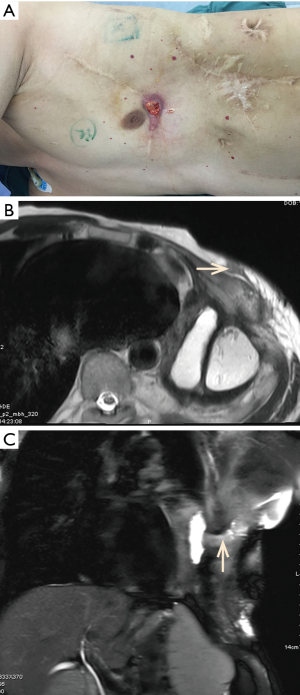
Later he received pedicle great omentum transfer for left thoracic empyema 7 years ago and pedicle latissimus dorsi muscle flap transfer for chest wall sinus 3 years ago. Unfortunately, a dead space remained in the left thoracic cavity after the surgery. Then he developed left chronic empyema and chest wall sinus. The activated partial thrombin time (APTT) was prolonged to 55.4 seconds (normal control 25.1 to 36.5 seconds), and the factor VIII activity was only 10%. Other laboratory tests were normal. Physical examination showed a 1 cm × 1 cm of chest wall sinus filled with fluids in one of scarred incisions in the left thorax (Figure 1A). Magnetic resonance imaging (MRI) of the chest revealed a 11.4 cm × 6.8 cm × 4.1 cm of empyema cavity connected to chest wall sinus (Figure 1B,C). During operation the patient was placed in the right decubitus position and 2,500 IU of recombinant coagulation factor VIII (Bayer HealthCare LLC, NY, USA) was infused. After the resection of chest wall sinus, scarred skin according to the location of the empyema space, two rib segments were resected to expose the entire cavity which filled with 100 mL of dark red pus (the pathogen identified on culture was proteus mirabilis). Then effusion inside the cavity was sucked out, the necrotic tissue around the cavity was removed and complete debridement was achieved. An ipsilateral thoracodorsal artery and two accompanying veins were prepared as the recipient vessels in the upper left thorax. Then a 27 cm × 11 cm of free vastus lateralis musculocutaneous flap was harvested from his left thigh and transferred to obliterate the residual space. Muscular part of the flap was placed directly upon the inner surface of empyema cavity. The descending branches of lateral circumflex femoral artery and two accompanying veins functioning as nutrient vessels were separately microvascular anastomosed end to end to the ipsilateral thoracodorsal vessels by interrupted suture with 9-prolene. After completion of the microvascular anastomosis, this free flap was firmly stabilized inside the space with surrounding tissue using monofilament absorbable sutures and the remaining skin paddle was sutured with surface skin.
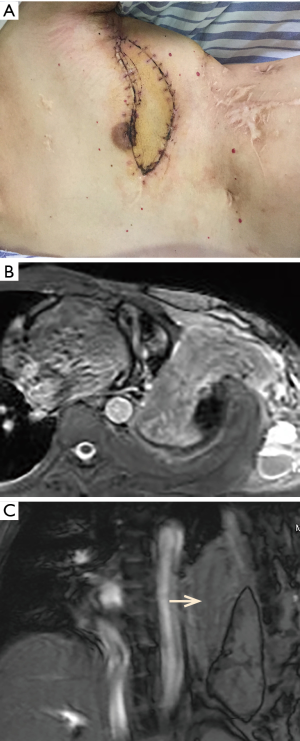
Donor site was closed primarily. The total blood loss was 1,200 mL, and 2 units of packed red blood cells and 300 mL of fresh frozen plasma were transfused. According to the frequently checked factor VIII levels, factor VIII was continued to use twice per day with 2,500 IU each time for a week after operation. Then the dose was gradually reduced. The drainage volume was about 1,060 mL hemorrhagic fluid on postoperative day 1. The chest drains were removed on postoperative day 10. The healing of incisions was without incident and the free flap was very well at discharge (Figure 2A). The chest MRI showed adequate obliteration of the thoracic space 3 months after surgery (Figure 2B,C). He had no recurrence of empyema and chest wall fistula during the 32 months of follow-up.
Discussion
Chronic empyema combined with chest wall sinus is generally regarded as a great challenge in clinical management. The difficulty exists not only in the fully control of the infection, but also in preventing dead space development (3,4). Meanwhile hemophilia A may produce a very high risk of excessive bleeding for a long time during and after surgery. There have been a few reports on cancer excision and coronary surgery which can be safely performed but no report on free musculocutaneous flap transfer for chronic empyema with chest wall sinus for these patients (1,2). Because it is necessary not only to complete obliterate infective dead space but also minimize the risk of surgical bleeding perioperation.
For the management of patients with hemophilia A, perioperative replacement of recombinant coagulation factor VIII is crucial for the safety of the major surgery such as cancer excision and coronary surgery (1,2,5,6). It is expected that 1 unit of factor concentrate per kilogram of body weight could increase the plasma factor VIII level by approximately 2 percent. The literature also recommended that the clotting factor VIII level should be maintained at 75 to 125 percent during surgery and for the first 24 hours after surgery, and above 50 percent during the first week after operation, so the dosage and duration of using coagulation factor VIII was determined by the type and extent of surgery (1,2,5,6). The treatment protocol for our patient was individualized to a dose of 2,500 IU of coagulation factor VIII twice per day for the first week after operation, then gradually reduced till withdrew according to frequently checked factor VIII levels. The stable coagulation factor VIII level minimized the risk of perioperative bleeding which ensured a good prognosis of the patient.
For the management of chronic empyema with chest wall sinus, several local pedicled muscular flaps or omental tissue may be used because they may provide a source of reliable vascularized tissue that enhances eradication of the infection and allows complete obliteration of the dead space, which promotes wound healing followed by gradual lung expansion (4,7). However, in our case, free musculocutaneous flap transfer is probably the last treatment option because his great omentum and the local flaps had been used previously and a large autologous tissue will be required to obliterate the empyema cavity (8-10). We harvested a free vastus lateralis musculocutaneous flap from his left thigh to completely obliterate the diseased space. Since the application of extrathoracic skeletal muscle into the pleural space was reported in 1989, an omental flap, rectus abdominis musculocutaneous flap, and latissimus dorsi musculocutaneous flap have been reported. Muscular tissue transfer eliminates dead space, brings vascularity to the surrounding tissues, and prevents bacterial inoculation (10). Comparing with other free musculocutaneous flaps such as rectus abdominis flap and latissimus dorsi flap, vastus lateralis flap is easier, and safer to be elevated and can provide a large bulk for obliteration of the diseased space. They contain better nutrient vessels to be microvascular anastomosed to the recipient vessels while minimizing the incidence of damage in the donor site (4). Postoperative chest MRI showed the free vastus lateralis musculocutaneous flap had been definitely complete obliteration of diseased space. He has no recurrence of empyema and chest wall fistula during postoperative follow-up. Here we conclude that a free vastus lateralis musculocutaneous flap transfer is safe and effective in management for refractory chronic empyema with chest wall sinus in a patient with hemophilia A when adequate factor activity is provided to achieve a safe hemostasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nomoto Y, Kimura H, Iwai N, et al. Completion pneumonectomy of the residual left lung to treat lung cancer in a patient with hemophilia A: report of a case. Surg Today 2000;30:917-20. [Crossref] [PubMed]
- Barillari G, Pasca S, Erice F, et al. Successful double bypass in a patient with severe hemophilia A: a case report. J Thromb Thrombolysis 2012;33:193-6. [Crossref] [PubMed]
- Walsh MD, Bruno AD, Onaitis MW, et al. The role of intrathoracic free flaps for chronic empyema. Ann Thorac Surg 2011;91:865-8. [Crossref] [PubMed]
- Tsai YT, Chen CC, Lu HI, et al. Free anterolateral thigh combined flap for chronic postpneumonectomy empyema. Ann Thorac Surg 2010;90:651-4. [Crossref] [PubMed]
- Eren A, Friedl R, Hannekum A, et al. Cardiac surgery in a patient with haemophilia A. Thorac Cardiovasc Surg 2006;54:212-4. [Crossref] [PubMed]
- Stine KC, Becton DL. Use of factor VIII replacement during open heart surgery in a patient with haemophilia A. Haemophilia 2006;12:435-6. [Crossref] [PubMed]
- Lu C, Feng Z, Ge D, et al. Pedicle muscle flap transposition for chronic empyema with persistent bronchopleural fistula: experience of a single clinical center in China. Surg Today 2016;46:1132-7. [Crossref] [PubMed]
- Molnar JA, Pennington DG. Management of postpneumonectomy bronchopleural-cutaneous fistula with a single free flap. Ann Plast Surg 2002;48:88-91. [Crossref] [PubMed]
- Okuda M, Yokomise H, Muneuchi G, et al. Obliteration of empyema space by vascularized anterolateral thigh flaps. Ann Thorac Surg 2009;87:1615-6. [Crossref] [PubMed]
- Sakuraba M, Umezawa H, Miyamoto S, et al. Reconstructive surgery for bronchopleural fistula and empyema: new application of free fascial patch graft combined with free flap. Plast Reconstr Surg Glob Open 2017;5. [Crossref] [PubMed]


