Chest wall resection and reconstruction for tumors: analysis of oncological and functional outcome
Introduction
Chest wall resection and reconstruction (CWRR) is required for a wide spectrum of diseases. Infiltrating non-small cell lung cancer (NSCLC), primary chest wall tumors, secondary malignancies, as well as infections and post-irradiation wounds, represent the most common settings for these procedures (1). In the treatment of primary and secondary tumors, the extent of the resection represents a crucial aspect for both radical resection and risk of instability of the bony chest after surgery (2). Radical resection is the key to the treatment. Postoperative morbidity has been mainly attributed to chest cage instability (3,4) but this issue is still a matter of debate, especially in view of the fact that there is no evidence regarding the impact of reconstruction, non-reconstruction and type of reconstruction in the functional outcome after the procedure. The correct surgical planning constantly requires soft tissue coverage and skin closure, while rigid stabilization of the bony thorax is not always necessary and represents a controversial issue. The aim of this study is to analyze our experience in order to ascertain the prognostic indicators and better understand when reconstruction of the thoracic wall is required and what the possible complications are for a non-reconstructed patient, after extensive demolition.
Methods
Retrospective analysis of a series of consecutive patients who underwent CWRR for neoplastic indication has been performed. Patients undergoing CWRR for chest wall metastasis, or for direct infiltration from malignancies other than NSCLC, or for non-neoplastic indication were not included in the analysis. Data were collected from inpatient hospital records and follow-up information from the referring oncologist.
The sample of this retrospective study is formed by 71 patients, 59 males and 12 females ranging from 18 to 82 years old [median (M) 64, standard deviation (SD) 11], who underwent thoracic demolition for tumors between April 2000 and October 2016. The follow-up ranged between 1 and 198 months (mean 52, M 37, SD 47).
Considering the heterogeneity of the population enrolled in this retrospective study, patients were divided into 2 groups, group 1: primary malignant tumors of the chest (17 patients); group 2: NSCLC infiltrating the chest wall (54 patients, 17 of whom were affected by Pancoast tumors).
Patients in both groups were preoperatively assessed by respiratory, cardiovascular and metabolic function tests. All cases underwent contrast-computed tomography (CT) of the thorax, abdomen and cranium; in addition, 10 patients underwent magnetic resonance imaging of the thorax and the dorsal column, in three cases pulmonary scintigraphy was performed and in 41 cases an 18-FDG positron emission tomography-CT total body was carried out in order to evaluate surgical indication. Every patient in group 2 underwent a pre-operative histological analysis of the tumor using trans-thoracic CT-guided needle aspiration, trans-thoracic echo-guided needle aspiration, trans-bronchial biopsy or EBUS-TBNA (Endobronchial ultrasound, transbronchial needle aspiration). In group 1, incisional biopsy was excluded from the work-up, due to the risk of tumor seeding. Needle aspiration was only performed in some patients in group 1 when deemed useful for the treatment strategy. The preoperative embolization according to the already described technique was carried out in 3 patients in group 1, in order to facilitated resection in giant hypervascular tumors (5). Each case was analyzed and discussed by a multidisciplinary dedicated team including oncologists, pulmonologists, radiotherapists and thoracic surgeons.
Nine cases were surgically managed by a multidisciplinary surgical team, one case in collaboration with neurosurgeons and eight cases in collaboration with the plastic surgery unit.
In our series, 33 patients underwent skeletal prosthetic stabilization, and in six of which prosthetic reconstruction and muscular flaps were combined. Thirty-six patients were not reconstructed and in two cases the reconstruction was achieved with muscular-flap alone without bony reconstruction.
According to the primary indication, the series of patients underwent different surgical interventions.
Group 1 (primary tumors of the chest wall): 14 of the 17 patients underwent prosthetic reconstruction of the thoracic defect. Among these 14 patients, 6 had prosthetic reconstruction alone, 6 had prosthetic and muscular flap reconstruction; while 2 patients were reconstructed by muscular-flap alone. Three patients underwent concomitant wedge resection of the infiltrated lung below the primary chest wall tumor. In 5 cases, a sternal resection was performed: 4 patients had a prosthetic reconstruction plus muscular flap and 1 patient underwent sternal allograft transplantation. Phrenoplasty was performed in 2 cases, in one of whom combined with a synthetic prosthesis and titanium bars. In two cases, the team decided to avoid bony reconstruction of the thorax after resection, considering the width and the site of the defect (small defects in non-critical areas).
Group 2 (NSCLC infiltrating chest wall): all patients in this group underwent major lung resection en bloc with the thoracic wall. In 2 patients, mediastinoscopy and in 12 EBUS-TBNA was performed, before the thoracotomy in order to rule out N2 disease. Nineteen of 54 patients underwent prosthetic reconstruction. No soft-tissue reconstruction by muscle flap was performed in this group. In 1 case, resection of a vertebral body was necessary, and carried out in cooperation with a neurosurgeon. In other 2 cases, resection of the vertebral transverse process and, in 7 cases, a complete disarticulation of the ribs from the vertebrae were performed. Seventeen Pancoast tumors were treated in this group: 10 posterior Pancoast tumors were treated through a posterior approach and 7 anterior Pancoast tumors were approached, either with a transmanubrial anterior approach (3 cases) or with a combined anterior and posterior approach (4 cases) (2). Histological findings are summarized in Table 1.

Full table
According to our criteria for reconstruction, we divided our experience in two categories based on the location and the width of the defect. The division of the chest into critical and non-critical areas based on the need of bony thorax reconstruction after resection, has already been described by some of us (2).
Defects that require skeletal reconstruction: resections in critical areas of the chest (anterior or lateral chest wall resection); defects of 3 or more ribs not covered by the scapula.
Defects that do not require skeletal reconstruction: small posterior rib resection of less than 3 ribs or any defect totally covered by the scapula.
Forty-one patients underwent chest wall demolition in critical areas while 30 patients underwent a chest wall resection in non-critical area. In both groups, we investigated several parameters: tumor stage (T and N parameters), free margins (R), infiltration depth of the tumor, number of resected ribs (width of the defect), location of the defect (critical or non-critical area), induction and/or adjuvant chemo and/or radiotherapy, 5-year and overall survival (OS), local or distant relapses and post-operative infections, chronic pain, flail chest, acute respiratory failure and cosmetic results.
Pathological staging of the disease was based on the 7th edition of the TNM classification.
Data were collected and retrospectively analyzed. IBM SPSS 23.0 (SPSS Inc., Chicago, Illinois) Windows software was utilized for the statistical analysis. Categorical data were compared with the T-student test and Exact Fisher test. OS and relapses were analyzed with the Kaplan-Meier test and Cox regression. Complications and 5-year survival were studied using linear regression and simple linear correlation. A P value of 0.05 or less was considered statistically significant.
Results
In the two groups, 86% of the patients were smokers; in group 2 this percentage reached 96%. Comorbidities: 28 patients were affected by hypertension, 7 by type II diabetes, 6 by chronic obstructive pulmonary disease, 8 by gastroesophageal reflux disease and 5 had a previous non-pulmonary carcinoma.
In 6 cases, a sternal resection was performed and in 4 of these cases a partial sternectomy en bloc with the ribs was carried out. The number of resected ribs varied between 2 and 6 (mean 3, 56% of patients). Free margins were obtained in 76% of the procedures (R0), microscopically involved margins were observed in 24% of the sample (R1) and no R2 disease was registered.
Relapses
Recurrence rate for the whole sample was 59%. Relapses were diagnosed between 2 and 126 months (mean 28, SD 32) and were directly correlated to R1 disease (P<0.05). Deep infiltration was not statistically significant (P=0.08).
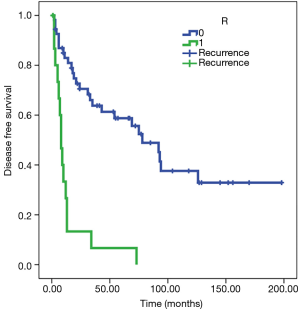
Patients with R0 resection are associated with a probability of recurrence after 97 months on average [standard error (SE) 12], while patients with R1 resection tend to show recurrent disease in 14 months on average (SE 4). The Cox regression model confirmed the differences in the risk of overall death between the two subgroups [hazard ratio (HR) 5.9, 95% CI: 2.945 to 11.929, P<0.001) (Figure 1).
Survival
Forty-five of 71 patients had already passed away by the time of follow-up (63% of the sample): in 34 cases, death was related to the disease, while in 11 cases death was related to other causes. No patient was lost at the follow-up time. The disease relapsed in 59% of patients.
The 5-year survival rate was 44% (evaluable in 56 patients): 67% in R0 and 15% in R1. Thus, an inverse correlation between R parameter and 5-year survival was detected (P<0.01).
Analyzing the OS, there is a strong correlation with the R parameter (P<0.01), with a prognosis of 95 months in patients R0 (SE 11) and 22 for R1 (SE 5) (Figure 2). The Cox regression model confirmed the differences in the risk of overall death between the two subgroups (HR 5.6, 95% CI: 2.814 to 11.047, P<0.001).

Oncological analysis in group 2
In group 2, 43 patients were T3 (79.6%); 43 patients were N0 (79.6%), 7 patients N1 and 3 patients N2 (for the statistical analysis, given the small sample of the N2 subgroup, N1 and N2 patients have been classified together in a single “N+” sample). Twenty-one patients underwent induction therapy with radiotherapy ± chemotherapy (RT ± CHT) (38.9%) and 46 underwent adjuvant therapy (85.2%).
Statistical analysis showed a significant direct correlation between relapse and the N parameter (P<0.01) and R parameter (P<0.05). The Kaplan-Meier test showed how in N0 patients, disease-free survival (DFS) was estimated to be 77 months (SE 13) and DFS was 8 months in patients N+ (SE 1); analyzing parameter R, DFS was estimated at 80 months for R0 patients (SE 14) and 10 months for R1 patients (SE 2) (Figures 3,4). Adjusted Cox regression showed a high risk of relapses for N+ patients (HR 2.8, CI 1.015 to 7.678, P<0.05) and for R1 patients (HR 3.6, 95% CI: 1.375 to 9.303, P<0.01).
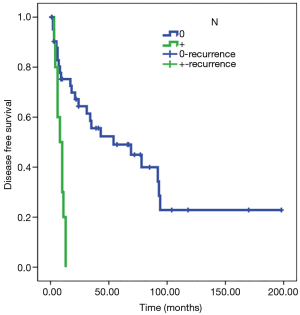
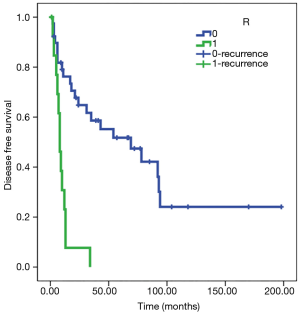
The T parameter resulted not to be statistical significant, probably because of the numerical exiguity of T4. No significant correlation between induction or adjuvant therapy and DFS was discovered in our series.
Five-year survival was assessed at 49% in group 2 and there was a significant inverse correlation with N+ and R1. OS only showed an inverse correlation with R1 (P<0.05).
The Kaplan-Meier test showed an OS of 78 months in R0 patients (SE 11) and 19 months in R1 patients (SE 4) (Figure 5). Cox regression confirmed the relation (HR 5.5, 95% CI: 2.469 to 12.225, P<0.001).
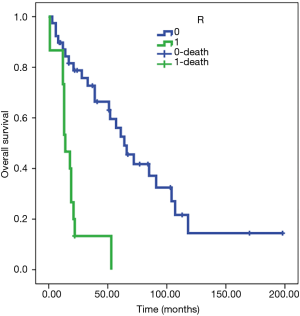
Peri-operative complications
One intra-operative complication was recorded: a ventricular fibrillation during surgery, promptly resolved. Early post-operative respiratory acute failure occurred in 10 patients; six of whom needed either a mini-tracheotomy, a standard tracheotomy or an orotracheal intubation and admission to the Intensive care unit. In this latter subgroup of patients, only 2/10 underwent chest wall reconstruction. Post-operative non-respiratory complications were recorded in 22 of 71 patients: atrial fibrillation, acute urinary retention, acute renal failure and 2 re-intervention for hemostasis. All patients recovered and no infection of the chest wall prosthesis was observed. In one case, we had a late complication: dislocation of a titanium bar after a mild trauma. Additional delayed complications were as follows: chronic pain in 15 patients, flail chest in 9 patients (6 of them non-reconstructed) and chest wall deformities in 14 patients.
A significant statistical correlation was found between the site of demolition (critical or non-critical areas) and the incidence of chest wall deformities and flail chest (P<0.05). Furthermore, multiple resected ribs (>3) influenced the incidence of deformities (P<0.01). Acute respiratory complications were also related to chest wall deformities, flail chest (P<0.01) and demolition without reconstruction (P<0.05) (Table 2).

Full table
In patients who underwent CWRR in a critical area, stabilization of the chest wall showed an inverse correlation (statistically significant) with acute respiratory complications, flail chest and deformities of the chest wall (P<0.01) (Table 3). Among patients submitted to chest wall resection in a critical area, 100% experienced a post-operative acute respiratory complication in the non-reconstructed subgroup, while only 5.7% in the reconstructed subgroup.

Full table
Discussion
CWRR represents a disputed topic regarding specific technical details and choices. The evolution in surgical techniques, prosthetic materials and oncological therapies, developed during the last century, led to a vast increase of surgical indications for tumors of the chest wall. The progress shown in the last decades has brought different treatment options and favorable multimodality treatment plans. CWRR are surgical procedures characterized by a potentially high rate of mortality, morbidity and peri-operative complications (6).
From a review of the related literature, the principal factor correlating with survival in patients affected by chest wall tumors is the “R parameter”. Our study confirmed the same correlation between free surgical margins and 5-year survival. OS and recurrence rate have shown to be correlated as well.
Primary tumors of the chest wall represent less than 5% of all thoracic malignancies, more frequently sarcomas (about 80%), with a high prevalence of chondrosarcoma (7). Free surgical margins are the main prognostic criteria; histology and differentiation grade of the tumor are considered second level prognostic factors (8). Indeed, at least 4 cm free surgical margin is recommended. R1 patients have to undergo re-resection, if possible, in order to achieve a R0 condition and therefore a better prognosis. Likewise, for such tumors, radical surgery is the only chance of cure, as well as for the rarer primary tumors often showing chemo and radio-resistance (9,10). Because of the wide resection required, patients with primary chest wall tumors frequently need complex reconstruction of soft tissues and bony thorax after surgery (8).
Surgery for patients with NSCLC infiltrating the chest wall are limited to N0–N1 disease, since patients with biopsy-proven N2 disease are not generally considered surgical candidates. Surprisingly, the T parameter and histological characterization seems to be prognostically less relevant. On the other hand, in addition to the N status, the vertebral and subclavian vessel involvement and the R parameter are considered negative prognostic factors. In the related literature, 5-year survival in patients operated for NSCLC infiltrating the chest wall ranges from 28% to 61% (for Pancoast tumor the values range from 44% to 56%) (11). Nodal involvement and complete resection are the main prognostic factors (11). For patients in our series (group 2) the 5-year survival was 49%. Statistical analysis in group 2 showed prognostic factors related to nodal involvement and presence of free margins to be significant for recurrences and also for 5-year survival, confirming the literature data, but, the OS only had a significant statistical correlation with the complete resection. In some studies, another relevant prognostic factor is represented by the infiltration depth of the tumor: it is considered superficial if only the parietal pleura is involved and deep if the infiltration involves the chest wall beyond the parietal pleura (12). We cannot confirm these data since our sample was composed only by patients who underwent CWRR, excluding extrapleural resections for minimal infiltration. The mortality rate after surgery for NSCLC infiltrating the thoracic wall is reported in the literature as 6% on average (11); in our series the mortality rate was 2.8%.
Indications to reconstruct the chest wall are still debated. In the literature, conclusions are not clearly established: the reconstruction rate varied from 40% to 60% (11), showing an important discrepancy among the different series in performing the reconstruction phase. A large trial on this topic is not available and the consequences on the respiratory function in patients submitted to wide chest wall resection without skeletal reconstruction have not been assessed (13). Some authors suggested not to stabilize the defect if it is located posteriorly with a maximum diameter of five centimeters or if the defect is covered by the scapula; however, they suggested reconstructing the chest wall if resection involves 3 or more ribs and the defect cannot be covered by the scapula (14,15). There is a general agreement that reconstruction is required when the tip of the scapula can be trapped into the lower margin of the defect. Our sample was divided into two different surgical subgroups: patients who underwent resection in critical areas (anterior or lateral resection, three or more ribs resected) and patients who underwent demolition in non-critical areas (posterior resection, less than three ribs resected or any defect covered by the scapula). Consequently, 49.3% of our patients were reconstructed. From the statistical analysis (involving both group 1 and 2), a correlation emerged between the demolition of chest wall in critical area and a higher percentage of flail chest, deformities and acute respiratory failure. In all of these patients, stabilizing the defect led to a significant reduction in the rate of the abovementioned complications and a much higher quality of life.
There are several choices in chest wall reconstruction and no single material or technique fulfill all the ideal features. The choice of the right method for the individual case is a matter of experience. In some patients, the problem of post-operative flail chest is attenuated, but not abolished after reconstruction with large non-rigid prostheses. Furthermore, the use of this kind of reconstruction after extensive thoracotomy can alter the anatomical shape of the chest profile, because the mesh must be placed under tension, stretched between the ribs bordering the defect, resulting in a sort of straight line, drawn between the rib stumps.
However, in our opinion, the use of a rigid prosthesis for chest wall reconstruction should be reserved only to the patients with very large defect in a “critical area” of the chest (2) when other simpler techniques of stabilization are considered inadequate. In fact, all the rigid materials show some drawbacks (potentially painful; not incorporable into the host tissue; dangerous in case of blunt trauma).
The herein reported clinical experience is also based on a previous variety of experimental research activities in this area concerning both the prosthetic materials and the techniques (16-20). In our study the criteria for chest wall stabilization were homogeneously adopted in all patients and this could be an interesting point for a comparative analysis with other similar experiences. The main limitation of our study is that it is a single-institution retrospective analysis.
Conclusions
Free surgical margins (for primary tumors), and free margins along with N0 status (for NSCLC infiltrating the chest wall) are the main oncological prognostic factors in patients undergoing chest wall resection for tumor. In our analysis of post-operative complications, in patients submitted to chest wall resection in critical areas, skeletal reconstruction positively influences the outcome, considerably reducing the respiratory complication rate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Santa Maria della Misericordia Hospital.
References
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Puma F, Vannucci J. Chest wall resection/reconstruction for tumors. In: Mathisen DJ, Morse CR, Fischer JE, editors. Master Techniques in Surgery. Thorac Surg Philadelphia: W Kluwer, 2015:312-58.
- Azoury SC, Grimm JC, Tuffaha SH, et al. Chest Wall Reconstruction: Evolution over a decade and experience with a novel technique for complex defects. Ann Plast Surg 2016;76:231-7. [Crossref] [PubMed]
- Hazel K, Weyant MJ. Chest wall resection and reconstruction: management of complications. Thorac Surg Clin 2015;25:517-21. [Crossref] [PubMed]
- Puma F, Cardini CL, Passalacqua G, et al. Preoperative embolization in surgical management of giant thoracic sarcomas. Eur J Cardiothorac Surg 2008;33:127-9. [Crossref] [PubMed]
- Spicer JD, Shewale JB, Antonoff MB, et al. The influence of reconstructive technique on perioperative pulmonary and infectious outcomes following chest wall resection. Ann Thorac Surg 2016;102:1653-9. [Crossref] [PubMed]
- Harati K, Kolbenschlag J, Behr B, et al. Thoracic wall reconstruction after tumor resection. Front Oncol 2015;5:247. [Crossref] [PubMed]
- Foroulis CN, Kelontas AD, Tagarakis G, et al. Massive chest wall resection and reconstruction for malignant disease. Onco Targets Ther 2016;9:2349-58. [PubMed]
- Thomas M, Shen KR. Primary tumors of the osseous chest wall and their management. Thorac Surg Clin 2017;27:181-93. [Crossref] [PubMed]
- Kachroo P, Pak PS, Sandha HS, et al. Single-Institution, multidisciplinary experience with surgical resection of primary chest wall sarcomas. J Thorac Oncol 2012;7:552-8. [Crossref] [PubMed]
- Filosso PL, Sandri A, Guerrera F, et al. Primary lung tumors invading the chest wall. J Thorac Dis 2016;8:S855-62. [Crossref] [PubMed]
- Lee CY, Byun CS, Lee JG, et al. The prognostic factors of resected non-small cell lung cancer with chest wall invasion. World J Surg Oncol 2012;10:9. [Crossref] [PubMed]
- Leuzzi G, Nachira D, Cesario A, et al. Chest wall tumors and prosthetic reconstruction: a comparative analysis on functional outcome. Thorac Cancer 2015;6:247-54. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- Puma F, Ragusa M, Daddi G. Chest wall stabilization with synthetic reabsorbable material. Ann Thorac Surg 1992;53:408-11. [Crossref] [PubMed]
- Puma F, Avenia N, Ricci F, et al. Bone heterograft for chest wall reconstruction after sternal resection. Ann Thorac Surg 1996;61:525-9. [Crossref] [PubMed]
- Puma F, Fedeli C, Ottavi P, et al. Laparoscopic omental flap for the treatment of major sternal wound infection after cardiac surgery. J Thorac Cardiovasc Surg 2003;126:1998-2002. [Crossref] [PubMed]
- Puma F, Vannucci J, Santoprete S. External longitudinal titanium support for the repair of complex pectus excavatum in adults. Eur J Cardiothorac Surg 2012;42:e166-8. [Crossref] [PubMed]
- Scarnecchia E, Liparulo V, Pica A, et al. Multidisciplinary approach to chest wall resection and reconstruction for chest wall tumors, a single center experience. J Thorac Dis 2017;9:5093-100. [Crossref] [PubMed]


