Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation
Introduction
Human spaceflight has undergone significant changes since its beginnings almost 50 years ago. With the establishment of the International Space Station (ISS) in low-Earth orbit (LEO), circling the earth at an altitude of around 400 km, crews sent into orbit have become larger and more heterogeneous and the duration of spaceflight has increased. To date, almost ninety crews have visited the ISS with a standard duration of each mission of around 6 months. Human missions beyond LEO refer to the Apollo Lunar programme, which was terminated in 1972, after a total of twelve astronauts had set foot on the surface of the Moon for a maximum of few days.
Today, human exploratory missions to the Moon or Mars, are widely considered as the next logical steps in human space exploration and, lately, colonisation. Almost all major national and international space agencies in the world as well as private investors and commercial initiatives are currently developing roadmaps and associated technologies to bring safely human beings to other planets in the solar system. Progressively, humanity shall get used to the concept of interplanetary travels among planets of our solar system, which shall become as natural as the concept of intercontinental flights on Earth. Human space flight will also see in the medium term a significantly increased number of travellers, longer durations of the flights, and longer distances up to stable permanence of human colonies on other planets. This will open to a number of challenges to be overcome, starting with ideological, but also political, technical, scientific, and even legal, philosophical, ethical and certainly medical.
The majority of the medical challenges faced during the human missions and associated durations performed so far, were mainly referring to radiations and micro- or reduced-gravity effects as well as psychological associated issues (1). Future exploratory missions to the Moon and Mars, including the establishment of permanently crewed base on the planet’s surface, will extend the distances travelled, the intensity of the radiation, the micro- and reduced-gravity levels, the duration of the mission, and the levels of confinement and isolation to which the crews will be exposed. This will raise several health issues which may be limiting factors during these missions, in particular radiation health, gravity related effects and psychological issues. Crew health and performance have to be ensured during transfer flights and planetary surface exploration, including Extra Vehicular Activities (EVAs), and upon return to Earth. Particularly a mission to Mars poses even further challenges: the distance of the planet, the duration of the trip (at least 500 days) and the impossibility to abort the mission lead to the necessity for the mission to be completely self-sustainable, since no support from Earth can be received in case of a health or major technical contingency.
In this scenario, 3D bioprinting for regenerative medicine and ultimately organs reproduction and transplantation is considered by the European Space Agency (ESA) a long term enabling technology for distant planet exploration and colonisation.
3D printing, or additive manufacturing (AM) includes a large family of processes and technologies and can be applied to a very wide range of materials, ranging from metals, polymers and ceramics but also living cells and organs (2-9). 3D bioprinting has already been used for the generation and transplantation of several tissues, including multilayered skin, bone, vascular grafts, tracheal splints, heart tissue and cartilaginous structures. Other applications include developing high-throughput 3D-bioprinted tissue models for research, drug discovery and toxicology.
ESA has pioneered in the exploration of the currently available Earth based 3D bioprinting technologies with the aim of defining a strategic roadmap for the safe, reliable and sustainable utilisation of these technologies on planet, aiming at exploration and colonisation missions support. ESA’s long-term vision is presented in the present work.
3D bioprinting—state of the art on Earth
Regenerative medicine and tissue engineering aim at the development of functional biological tissue substitutes enhancing tissue reconstruction and regeneration. Alternatively, transplantation of stem cells is a promising approach with the potential of self-renewal and differentiation. The so called stem cells niche, is the complex 3D environment which influences the cells fates. Within this system cell-cell contacts, cell-matrix adhesion, and the exchange of growth factors and oxygen are required for stem cells regulation (10). Therefore the proper fabrication of niche-like environment is a key issue in stem cell biology and regenerative medicine (10-12). The production of stem cell niches and tissue constructs is very challenging from a technical point of view with respect to their complexity. A technology is needed that allows the generation of defined 3D microstructures copying original tissues templates. By this means there is a demand to develop natural substitutes instead of traditional 3D scaffolds (13,14), which often proved to be challenging since they limit oxygen exchange within the tissue. Moreover, the structure has to be formed out of a material that is not only compatible with the cells but also enables the exchange of nutrient and soluble factors. Further, material elasticity and forces have to be taken into account since these parameters are known to influence stem cells differentiation. Also, the cells have to be arranged in 3D, enabling the formation of close cell-cell as well as cell-matrix contacts and interactions. Lastly, cell differentiation has to be guided and controlled within this complex 3D system. As differentiation is dependent on the initial cells density, the cells constructs have to be formed out of a defined, variable, and high cells amount (15,16).
3D bioprinting is an AM technology, where cells and biomaterials such cytocompatible hydrogel precursors, often referred as bioink, are simultaneously deposited in a layer by layer manner to generate biologically active 3D tissues of predesigned shape and size. Different cells types can be placed at desired locations of the bioprinted element and high cells densities can be achieved (17-19). The bioink properties before, during and after gelation are essential to its printability, and are impacting the achievable structural resolution, the shape fidelity and the cell survival. However, it is the final properties of the matured bioprinted tissue construct or niche that are crucial for the end application. During tissue formation these properties are influenced by the amount of cells present in the construct, their proliferation, migration and interaction with the material.
During the last decades, computer-aided deposition of biological materials has been investigated as a potential technique for engineering of tissue regeneration or replacement. A number of very comprehensive reviews have been published and summarise all relevant technologies, possible materials and associated benefits and limitations (11,19-37). In general, all existing bioprinting approaches can be separated into three groups, as sketched in Figure 1, including inkjet bioprinting (piezoelectric and thermal), orifice-free bioprinting (laser-induced forward transfer, LIFT, and printing by surface acoustic waves) and extrusion bioprinting (pneumatic or mechanical).
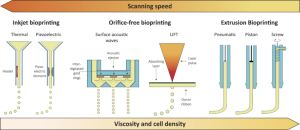
Inkjet bioprinting
An inkjet bioprinter delivers small droplets of bioink (1–100 picoliters; 10–50 µm diameter) on predefined locations of a substrate. The two most common methods used for inkjet printing of cells are piezoelectric and thermal inkjet bioprinting (23,38). The piezoelectric inkjet printer uses piezoelectric crystals to produce acoustic waves forcing small amounts of liquid through the nozzle. The thermal inkjet system produces pulses of pressure by vaporising the bioink around the heating element and expelling the droplets out of the printing head. Inkjet bioprinters are successfully applied with a micrometer resolution (10–50 µm) for the deposition of cells and are compatible with a number of bioinks (38-40). However, the major drawback of this technology is the achievable low viscosity and low cells density (41,42), since high cells density and associated high viscosity of the bioinks could result in clogging of the printing head. On the other hand, high scanning speeds can be achieved.
Orifice-free bioprinting
Orifice free bioprinting can be further divided into laser-induced forward transfer (LIFT) and surface acoustic waves printing. In LIFT, a pulsed laser beam is focused and scanned over a donor substrate coated with an absorbing layer (e.g., titanium or gold) and a bioink layer (11,37,43-46). Focused laser pulses cause local evaporation of the absorbing layer thereby creating a high-pressure bubble propelling small portions of the bioink towards the collector platform. Since this bioprinting technique is orifice free, it is not affected by clogging problems. Very high precision can be achieved combined with high cells survivability, since shear stress and extrusion are avoided. The resolution is in the range of 10–100 µm and bioinks with a viscosity ranging from 1 to 300 mPa and medium cells densities of ~108 cells mL-1 can be printed (31,47,48). The reduced scanning speed is the limiting factor of this technology.
Surface acoustic waves is the other orifice-free bioprinting method (49,50). Acoustic waves are produced by an acoustic ejector which uses a surface acoustic wave piezoelectric substrate (e.g., quartz, lithium niobate, etc.) with interdigitated gold rings placed on top of the substrate.
The waves have a circular geometry, hence an acoustic focal plane is generated at the air-liquid interface in the microfluidic channel and bioink droplets are ejected from it. The diameter of the droplets is uniform and ranges from 3 to 200 µm by tuning the wavelength of the acoustic ejector. High cells viability is achieved and bioinks with various surface tensions and viscosities can be ejected and processed.
Extrusion bioprinting
Extrusion bioprinting is probably the most common method for the fabrication of 3D cell-laden constructs (51-53). The bioink is normally inserted in disposable plastic syringes and dispensed either mechanically or pneumatically (piston or screw based) on the receiving substrate. This technology releases rather large amount of hydrogel filaments in the order of 150–300 µm in diameter. High viscosity and cells densities can be achieved with this method, though with an increased risk of cells damage due to the mechanical interaction with the orifice and the associated shear stress. Nozzle clogging and a reduced resolution (in the order of 200–1,000 µm) are the recognised drawbacks of the process. A new bioprinting approach is now proposed to improve the achievable environment for cells migration and spreading, currently suboptimal (54,55). In particular, a gel-in-gel bioprinting method bioink is extruded into a volume of self-healing hydrogel acting as a support material. The support hydrogel deforms after the injection of the bioink and heals immediately repairing and enclosing the printed structure inside. This method opens to multi-material printing, improves the mechanical properties of the construct and results in high cells viability.
Bioprinting has emerged in recent years as an attractive method for engineering of 3D tissues and organs in the laboratory, which can subsequently be implemented in a number of regenerative medicine applications. Currently, the primary goals of bioprinting are to (I) create complete replacements for damaged tissues in patients and (II) rapidly fabricate small-sized human-based tissue models or organoids for high-throughput diagnostics, pathology modelling, and drug development, examples of which are reported in Figure 2.
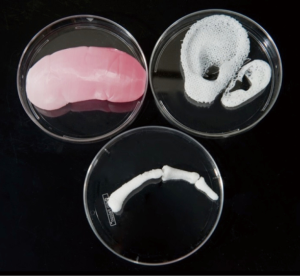
3D bioprinting has already been used for the generation and transplantation of several tissues, including multilayered skin, bone, vascular grafts, tracheal splints, heart tissue and cartilaginous structures. However, the capability of bioprinting fully functional complex tissues and organs still imply the resolution of a number of technical challenges (including e.g., the vascularisation, cells stability, production time and associated costs, capabilities of printing multi-materials, resolution vs. size of the print, cells viscosity and number, etc.), and addressing them requires the integration of technologies from the fields of engineering, biomaterials science, cell biology, physics and medicine.
ESA is considering this technology as a key enabler for long term/long distance missions, including planetary exploration and colonisation. This is particularly the case for the missions where the link with Earth will not be possible anymore and full mission self-sustainability shall be guaranteed.
Why other planets?
After the realisation of the ISS, human exploratory missions to the Moon or Mars, are widely considered as the next logical steps in human space exploration and, lately, colonisation. The ISS is the first example of an international cooperation for the joint development, operation and utilisation of a permanent space habitat in LEO. Hence, with the ISS a new era of peaceful cooperation in space on a global scale has started. Major partners are the USA, Russia, Japan, Europe and Canada. The experience matured with the ISS has shown that human beings can live, operate and perform science in space and that this can be done peacefully. The next logical step is to build on the technological, human, scientific and political lessons learnt matured in this endeavour and start bringing humanity to live, operate and perform science in other planets and in a permanent manner. The benefits and opportunities of exploring and eventually establishing permanent outposts on other planets (e.g., the Moon and Mars) are tremendous as described in (56) and reported here. They include:
- Determine if life is or was present outside of Earth and understand the environments that supported or could support it;
- Extend the human presence, exploring a variety of destinations beyond LEO with a focus on continuously increasing the number of individuals than can be supported at these destinations, the duration of time that individuals can remain at these destinations, and the level of self-sufficiently;
- Develop the necessary level of exploration technologies and capabilities: this shall include the knowledge, the capabilities, and the infrastructure required to live and work at destination beyond LEO through development and testing of advanced technologies, reliable systems, and efficient operations scenarios in an off-Earth environment;
- Perform science to support human exploration. Reduce the risk and increase the productivity of future missions in our solar system by characterising the effect of the space environment on human health and exploration systems;
- Provide other survivability options and resources to humankind beyond Earth;
- Stimulate economic expansion. Support and encourage the provision of technology, systems, hardware and services for commercial entities and create new markets based on space activities that will return economic, technological, and quality-of-life benefits for the whole humankind;
- Perform space, Earth and applied science. Engage in science investigations of, and from, solar system destinations and conduct applied science in the unique environment of solar system destinations;
- Provide opportunities for the public to engage interactively in space exploration;
- Enhance Earth safety. Enhance the safety of Earth by understanding the degradation processes of other (similar) planets, by following collaborative pursuit of planetary defence and orbital debris management mechanisms.
In support of the vision described above, a number of activities are ongoing and are currently paving the way for human planetary exploration and colonisation. In the following a number of the most relevant developments presently supporting the realisation of long term stable mission on the Mars surface are briefly described.
The recent decision by the ISS partners to extend its life until at least 2020 ensures that the ISS can be effectively further used to prepare for exploration, covering human science, engineering, biology and chemistry.
The current ongoing development of the Multi-Purpose Crew Vehicle (MPCV), part of the Orion program. Orion is NASA’s next spacecraft to send humans into space (Figure 3).

It is designed to send astronauts further into space than ever before, beyond the Moon to asteroids and even Mars. ESA has designed and is overseeing the development of Orion’s service module, the part of the spacecraft that supplies air, electricity and propulsion. Much like a train engine pulls passenger carriages and supplies power, the European Service Module will take the Orion capsule to its destination and back.
The intention to realise the Deep Space Gateway (Figure 4). The project envisions a modular spacecraft with an open architecture orbiting the Moon in the mid 2020s. Acting as a hub in the lunar vicinity, the platform is intended to be a staging post to multiple destinations. It will launch missions to the lunar surface, into deep space and ultimately to Mars (58).

Robotic missions have always served as the precursors to human exploration missions. Precursor robotic missions are essential to ensure human health, safety and the success of human missions and ensure maximum return of investments required for subsequent human exploration. A completely European manufactured rover (Figure 5), is part of the 2020 mission of the ExoMars programme that will also deliver a Russian surface platform to Mars. The rover will be the first mission to combine the capability to move across the surface and to study Mars at depth. It will collect samples with a drill down to a depth of 2 m and analyse them with next-generation instruments in an onboard laboratory. Underground samples are more likely to include biomarkers, since the tenuous martian atmosphere offers little protection from radiation and photochemistry at the surface. The primary objective is to land the rover at a site with high potential for finding well-preserved organic material, particularly from the very early history of the planet. The rover is expected to travel several kilometres during its mission.

ESA is also currently working on the building blocks technologies which will be required for the realisation of stable and sustainable human outposts on the Moon (57,59) and Mars (60), leveraging and maximising in-situ resources utilisation (ISRU) and mission self-sustainability.
The described missions constitute highly relevant technology building blocks for the development of a stable base on the Moon or on Mars.
However, for the realisation of a mission of these proportions the first challenge to be solved will be mainly a political one. Seen the level of financial, technological, scientific effort required, consensus shall be established among a wide range of nations, including Europe, the USA, Russia, but also China, India, Japan, Canada. Moreover, partnerships shall be established not only among institutional national and international space agencies, but it shall also be extended to the private sectors, where commercial initiatives are blossoming.
The space medical challenges and possible mission scenarios
A number of significant medical challenges are associated with human spaceflight and are linked to a set of factors, starting with the distance and the duration of the space journey. In particular the following issues play a major role in astronauts’ health during space flight:
- Radiation exposure (in terms of intensity, type of the radiating particles and irradiation time), mainly leading to neoplastic risk, central nervous system effects, cataracts, circulatory diseases and acute radiation syndromes (61-66);
- Reduced gravity levels, mainly leading to loss of muscles/bones mass, reduced cardiovascular activity, haematopoiesis and lymphopoiesis decrease, changing of motorial functions, visu’s reduction, wound healing capabilities reduction;
- Injures and burns due to missions’ hazards and contingencies;
- Psychological aspects.
In Table 1, a more detailed list of general health challenges and the associated probabilities during three different mission scenarios and durations to the Moon and to Mars is presented (1).
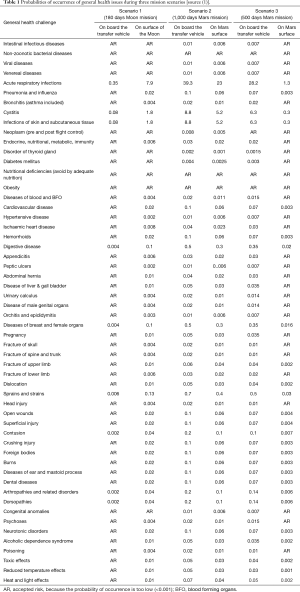
Full table
For the current missions’ scenarios and durations, mainly referring to the ISS in LEO, the above listed medical issues are mitigated by dedicated countermeasures, including:
- Materials for radiation protection are used on the ISS structures;
- Definition of radiation thresholds (and associated flight limitations for astronauts exceeding them) based on blood generating organs levels;
- Physical training of astronauts during their permanence in space.
Medical contingencies on-board of the ISS are currently treated as first-aid interventions. All astronauts are trained to perform first-aid and patient stabilisation. No surgery is performed, but all serious patients can be stabilised and reach a hospital on Earth for further specialised treatment within 20 hours.
A similar approach will be adopted for medical issues occurring on a Moon base, where no surgery will most probably be performed. Also, in this case, seriously ill astronauts will be returned to Earth’s based hospitals which can be reached in 3 to 4 days. Moreover, the establishment of a permanent Moon base will offer further opportunities to mitigate the radiations associated risks as well as the reduced gravity related issues. In particular, the Base structure can be manufactured such that radiation shielding is provided by the base walls and water present in their interstices. Moreover, dedicated physical training centres can be foreseen to counteract reduced gravity effects on human bones and muscles experience in the Moon’s reduced gravity.
If humans have to travel farer distances from the Moon and establish Mars permanent bases, different mission scenarios and strategies shall be envisaged. The Mars distance and the associated mission’s duration (at least 500 days) with no abortion possibility (due to astrodynamics conditions during the flight) will not allow the travellers with serious medical contingencies to be safely returned to Earth in reasonable time. Regenerative medicine and potentially 3D bioprinting shall be adopted (67-69), depending on mission duration and medical personnel and support infrastructure availability.
In particular, the following Mars mission scenarios can be anticipated:
- first Mars mission:
- Preliminary exploratory mission of short duration (500 days);
- Limited crew (4 to 5 astronauts);
- Medical surgeon on board with generic and broad capabilities;
- Although astronauts will be selected based on their strong medical health and genetics, skin burns and bones injures may occur due to mission contingencies;
- Hence, 3D bioprinting of skin and bones shall be considered however medical personnel and infrastructure limitations will confine its applications to small and superficial interventions;
- long term Mars mission/settlement:
- Longer term Mars missions up to human stable settlement (from 2 to 5 years and above);
- Larger crew (more than twenty astronauts);
- Medical and surgical infrastructure available;
- Generalist surgeon trained based on a surgical skills maintenance program using tele-training capabilities and augmented reality (telemedicine not possible due to Earth/Mars data transmission delay);
- 3D bioprinting capabilities considered mission enabling, for applications ranging from skin and bones up to tissues and organs implantation;
- Long term vision includes CT scanning of astronauts organs and stem cells support for organ reproduction in case of damage/failure on planet.
Adopting 3D bioprinting on a planet or in space (ideally also during the travel to reach the target planet) implies significant further challenges to be solved, with respect to the already open ones on Earth. In particular, the detrimental effects of radiation and microgravity on both the patients to be treated as well as the stem cells. Also, microgravity and/or reduced gravity will limit the bioprinting capabilities of the printer itself. Moreover, bio-printer shall be used in conjunction with sterile production areas, cells culturing and incubators systems as well as dedicated surgical infrastructures. Also specific pharmacological treatment ad or anaesthesia may be designed based on resources available on planet.
ESA has pioneered the addressing of all the above listed challenges within its vision of adopting 3D bioprinting and regenerative medicine for long term planet colonisation missions. In particular, the Agency has just kicked off the first study in this domain in which the following aspects will be analysed and consolidated (70):
- The definition of a concept for the utilisation (including clinical scenarios) of 3D bioprinting of living tissues as a technology to sustain life on long term/long distance human exploration missions and planetary colonisation;
- The definition of the requirements for 3D bioprinting of living tissues in the frame of manned space missions and planetary colonisation;
- The discussion of key domains (e.g., sensitivities of living cells such as printing parameters and conditions, materials selection, cell types etc.) of relevance for 3D bioprinting of living tissues but also specific to in-space/in-planet utilisation (e.g., covering radiation, micro- and reduced-gravity, limited resources availability, etc.) to be further investigated;
- The discussion of solutions and technologies (existing or to be developed);
- The identification of potential applicable regulations and norms (including safety aspects) regarding the performance of 3D bioprinting in space;
- The discussion of a preliminary accommodation concept for the realisation of an operating theatre in a space environment for 3D bioprinting and surgical implantation;
- The manufacturing of a tissue demonstrator, to be performed under space/planet representative conditions.
Conclusions
In the present paper, ESA’s vision related to the implementation of 3D bioprinting technologies for long-term/long-distance human space missions has been presented, and the following can be summarised:
- humanity has the opportunity for the first time in its history to fly farer in the solar system than ever before, but also it has the potential technologies for establishing permanent outposts on the Moon and on Mars surfaces;
- if a Mars outpost is conceived, the nature of the mission (including distance from Earth, impossibility of mission abortion during flight to and/or from planet) requires a fully self-sustainable mission concept, especially with respect to medical aspects;
- once appropriate medical and surgical infrastructure can be guaranteed on the planet surface, ESA considers 3D bioprinting as mission enabling, for applications ranging from skin and bones up to tissues and organs implantation;
- ESA is pioneering in addressing all associated challenges with the vision of safe, reliable and sustainable utilisation of 3D bioprinting and regenerative medicine for long term planet colonisation missions in the first exploratory study of this kind in Europe (70);
- the benefit of space research and technology developments may stimulate, as it is often the case in other scientific areas (71), an increased sensitisation and return of investment on Earth based development.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lasseur C. HUMEX. A Study of the Survivability and Adaptation of Humans to Long-Duration Exploratory Missions. ESA-SP 2003.1264.
- Ghidini T, Pambaguian L, Blair S. Joining the Third Industrial Revolution: 3D Printing for Space. Esa Bulletin 2015;163:24-33.
- Ghidini T. The Use of 3D Printing for Space Applications. J Space 2016;1:54-8.
- Ghidini T. The Potential of Additive Manufacturing (AM) for Space Technology. Proceedings of the International Laser Symposium, Key Note, Dresden, Germany, 2016.
- Brandão AD, Gerard R, Gumpinger J, et al. Challenges in Additive Manufacturing of Space Parts: Powder Feedstock Cross-Contamination and Its Impact on End Products. Materials (Basel) 2017.10. [PubMed]
- Martin-Iglesias P, Van Der Vorst M, Gumpinger J, et al. ESA's recent developments in the field of 3D-printed RF/microwave hardware. 2017 11th European Conference on Antennas and Propagation, 2017.
- Romano S, Brúckner-Foit A, Brandão A, et al. Fatigue properties of AlSi10Mg obtained by additive manufacturing: Defect-based modelling and prediction of fatigue strength. Eng Fract Mech 2018;187:165-89. [Crossref]
- Brandão AD, Gumpinger J, Gschweitl M, et al. Fatigue Properties Of Additively Manufactured AlSi10Mg – Surface Treatment Effect. Procedia Structural Integrity 2017;7:58-66. [Crossref]
- Romano S, Beretta S, Brandão A, et al. HCF resistance of AlSi10Mg produced by SLM in relation to the presence of defects. Procedia Structural Integrity 2017;7:101-8. [Crossref]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009;324:1673-7. [Crossref] [PubMed]
- Gruene M, Deiwick A, Koch L, et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods 2011;17:79-87. [Crossref] [PubMed]
- Ma T, Grayson WL, Fröhlich M, et al. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 2009;25:32-42. [Crossref] [PubMed]
- Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 2008;17:467-79. [Crossref] [PubMed]
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature 2004;428:487-92. [Crossref] [PubMed]
- Hui TY, Cheung KM, Cheung WL, et al. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials 2008;29:3201-12. [Crossref] [PubMed]
- Takagi M, Umetsu Y, Fujiwara M, et al. High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J Biosci Bioeng 2007;103:98-100. [Crossref] [PubMed]
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773-85. [Crossref] [PubMed]
- Hölzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016;8. [Crossref] [PubMed]
- Mironov V, Reis N, Derby B. Review: bioprinting: a beginning. Tissue Eng 2006;12:631-4. [Crossref] [PubMed]
- Chrisey DB. Materials Processing: The Power of Direct Writing. Science 2000;289:879-81. [Crossref] [PubMed]
- Hon KK, Li L, Hutchings IM. Direct writing technology-advances and developments. CIRP Ann Manuf Technol 2008;57:601-20. [Crossref]
- Calvert P. Materials science. Printing cells. Science 2007;318:208-9. [Crossref] [PubMed]
- Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther 2007;7:1123-7. [Crossref] [PubMed]
- Boland T, Xu T, Damon B, et al. Application of inkjet printing to tissue engineering. Biotechnol J 2006;1:910-7. [Crossref] [PubMed]
- Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016;102:20-42. [Crossref] [PubMed]
- Chang CC, Boland ED, Williams SK, et al. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 2011;98:160-70. [Crossref] [PubMed]
- Jakab K, Norotte C, Marga F, et al. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010;2. [Crossref] [PubMed]
- Malda J, Visser J, Melchels FP, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013;25:5011-28. [Crossref] [PubMed]
- Masters KS, Shah DN, Leinwand LA, et al. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005;26:2517-25. [Crossref] [PubMed]
- Pereira RF, Bartolo PJ. 3D bioprinting of photocrosslinkable hydrogel constructs. J Appl Polym Sci 2015;132:42458. [Crossref]
- Barron JA, Wu P, Ladouceur HD, et al. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices 2004;6:139-47. [Crossref] [PubMed]
- Jungst T, Smolan W, Schacht K, et al. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem Rev 2016;116:1496-539. [Crossref] [PubMed]
- Nakamura M, Kobayashi A, Takagi F, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng 2005;11:1658-66. [Crossref] [PubMed]
- Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008;29:193-203. [Crossref] [PubMed]
- Ringeisen BR, Othon CM, Barron JA, et al. Jet-based methods to print living cells. Biotechnol J 2006;1:930-48. [Crossref] [PubMed]
- Mironov V, Visconti RP, Kasyanov V, et al. Organ printing: tissue spheroids as building blocks. Biomaterials 2009;30:2164-74. [Crossref] [PubMed]
- Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855-63. [Crossref] [PubMed]
- Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. Int Mater Rev 2014;59:430-48. [Crossref]
- Summer R, Fine A. Mesenchymal progenitor cell research: limitations and recommendations. Proc Am Thorac Soc 2008;5:707-10. [Crossref] [PubMed]
- Xu T, Jin J, Gregory C, et al. Inkjet printing of viable mammalian cells. Biomaterials 2005;26:93-9. [Crossref] [PubMed]
- Calvert P. Inkjet printing for materials and devices. Chem Mater 2001;13:3299-305. [Crossref]
- Kim JD, Choi JS, Kim BS, et al. Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer 2010;51:2147-54. [Crossref]
- Guillemot F, Souquet A, Catros S, et al. Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine (Lond) 2010;5:507-15. [Crossref] [PubMed]
- Guillotin B, Guillemot F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 2011;29:183-90. [Crossref] [PubMed]
- Hopp B, Smausz T, Kresz N, et al. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng 2005;11:1817-23. [Crossref] [PubMed]
- Koch L, Kuhn S, Sorg H, et al. Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods 2010;16:847-54. [Crossref] [PubMed]
- Duocastella M, Colina M, Fernandez-Pradas JM, et al. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl Surf Sci 2007;253:7855-9. [Crossref]
- Guillemot F, Souquet A, Catros S, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 2010;6:2494-500. [Crossref] [PubMed]
- Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol 2013;31:10-9. [Crossref] [PubMed]
- Li C, Faulkner-Jones A, Dun AR, et al. Rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew Chem Int Ed Engl 2015;54:3957-61. [Crossref] [PubMed]
- Smith CM, Stone AL, Parkhill RL, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng 2004;10:1566-76. [Crossref] [PubMed]
- Duarte Campos DF, Blaeser A, Weber M, et al. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2013;5. [Crossref] [PubMed]
- Tan EY, Yeong WY. Concentric bioprinting of alginate-based tubular constructs using multi-nozzle extrusion-based technique. Int J Bioprint 2015;1:49.
- Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater 2011;23:H178-83. [Crossref] [PubMed]
- Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater 2015;27:5075-9. [Crossref] [PubMed]
- ISECG, The Global Exploration Roadmap. International Space Exploration Coordination Group, 2011.
- Cesaretti G, Dini E, De Kestelier X, et al. Building components for an outpost on the Lunar soil by means of a novel 3D printing technology. Acta Astronautica 2014;93:430-50. [Crossref]
- Carpenter J. ESA Workshop: Research Opportunities on the Deep Space Gateway. Available online: http://exploration.esa.int/moon/59392-esa-workshop-research-opportunities-on-the-deep-space-gateway/
- Woerner J, Foing B. The "Moon Village" Concept and Initiative. Annual Meeting of the Lunar Exploration Analysis Group, 2016.
- Buchner C, Pawelke RH, Schlauf T, et al. A new planetary structure fabrication process using phosphoric acid. Acta Astronautica 2018;143:272-84. [Crossref]
- Cucinotta FA, Cacao E. Non-Targeted Effects Models Predict Significantly Higher Mars Mission Cancer Risk than Targeted Effects Models. Sci Rep 2017;7:1832. [Crossref] [PubMed]
- Cucinotta FA, Alp M, Sulzman FM, et al. Space radiation risks to the central nervous system. Life Sci Space Res 2014;2:54-69. [Crossref]
- Chylack LT Jr, Peterson LE, Feiveson AH, et al. NASA study of cataract in astronauts (NASCA). Report 1: Cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat Res 2009;172:10-20. [Crossref] [PubMed]
- Cucinotta FA, Manuel FK, Jones J, et al. Space radiation and cataracts in astronauts. Radiat Res 2001;156:460-6. [Crossref] [PubMed]
- Cucinotta FA, Hamada N, Little MP. No evidence for an increase in circulatory disease mortality in astronauts following space radiation exposures. Life Sci Space Res (Amst) 2016;10:53-6. [Crossref] [PubMed]
- Little MP, Azizova TV, Bazyka D, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120:1503-11. [Crossref] [PubMed]
- Ghidini T. Technology (and Human) Challenges for Space Exploration and Colonisation. Proceedings of the IRCCS Policlinico San Donato Research Day, Plenary Lecture, San Donato, Italy, 2017.
- Ghidini T. Technology for Space and In-Space (and on-Earth) Applications. Proceedings of the XXVIII Meeting of the Italian Society for Cardiac Surgery, Plenary Lecture, Rome, Italy, 2016.
- Ghidini T. New Materials to Improve Efficacy and Safety: Lessons Learnt from the European Space Agency. Proceedings of the SICCH 50th Anniversary: The Excellence of Italy in Cardiovascular Diseases, Keynote Lecture, Rome, Italy, 2017.
- 3D Printing of Living Tissues for Space Exploration. Available online: http://www2.rosa.ro/index.php/en/esa/oferte-furnizori/2287-3d-printing-of-living-tissues-for-space-exploration-expro-plus
- Ghidini T. The ESA Advanced Manufacturing Cross-Cutting Initiative: An Opportunity to Raise European Technology Competitiveness. The Quarterly Bulletin of the CEAS, 2nd Quarter 2017.


