How to do it: tips and tricks of minimal-invasive HVAD® implantation—the lateral approach
Introduction
Chronic end-stage heart failure (CHF) is a major healthcare problem with rapidly growing incidences in Europe and the United States (1). With decreasing cardiac transplant rates worldwide, alternative treatment methods become increasingly important. The role and application of mechanical circulatory support (MCS) devices such as left ventricular assist devices (LVADs) for the therapy of CHF has increased worldwide (2-5). Novel technologies and surgical experience have played a key role in VAD-related successes. The most significant improvement in LVADs has been their essential miniaturization, which allows their implantation with less invasive surgical procedures (2-4,6-10). It is already known that minimally invasive surgical (MIS) procedures result in reduced wound size, ameliorated blood loss, shorter hospitalization duration, and improved cost-effectiveness. The reduction in post-operative complications has contributed to improved survival rates. This has led to considerably improved long-term outcomes over time (6,7,9-12). With further miniaturization of LVADs, the application of MIS techniques for implantations is likely to increase, giving rise to the need for well-established surgical approaches. The start of the development of the MIS-LVAD implantation technique for the HVAD as well as for other devices was worldwide first performed at Hannover Medical School in 2011 (2). The publication of this novel technique is the mark of a clinical paradigm shift in the therapy of patients with permanent ventricular assist devices. The implantation technique includes an upper hemi-sternotomy combined with anterior-lateral thoracotomy and is developed to reduce complication rates (trauma size, postoperative blood loss, right heart failure), thereby allowing earlier recovery and better survival. Several modifications of this approach have been described for LVAD implantation (2,6,7,13-15). HVAD implantation with this technique is acknowledged worldwide as a procedure to treat left ventricular failure (2,6). The HeartWare ventricular assist device or HVAD (Medtronic, Minnesota, MN, USA) is a miniaturized, continuous-flow, centrifugal, implantable VAD (16). The inflow is in the apex of the left ventricle and the outflow on the aorta ascendens, while the pump is operated by a percutaneous driveline. The current article describes the technical aspects and characteristic features of less invasive HVAD implantation in detail.
Surgical technique
The less invasive approach for HVAD implantation was developed at the Hannover Medical School, Germany in 2011 (2). Sometimes referred to as the “Hannover technique” this technique was introduced by Schmitto et al., and it is a combination of an upper mini-sternotomy and a left-sided anterolateral mini-thoracotomy (2).
At the start of the procedure, general anesthesia and single-lumen endotracheal intubations are performed, followed by an upper J hemisternotomy into the right second-to- third intercostal space (Figure 1). By obviating a total sternotomy the pericardium stays primarily unopened (only opened near the ascending aorta) in turn leading to additional right ventricular stabilization along with avoidance of right ventricular dilation during operation, in particular during the onset of the LVAD implantation. Thus, the right ventricular function remains passively sustained (2).
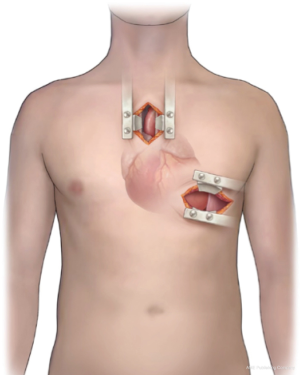
Thereafter, transthoracic echocardiography is performed for assessing the LV-apex. An anterior left 7–9 cm mini-thoracotomy through the fifth intercostal space is performed to access the apex cordis. A transesophageal echocardiography is performed while poking the apex to find the right place for the epicardial sewing ring of the HVAD onto the left ventricle. Soft tissue and Tuffier retractors are used to secure incisions. The HVAD-sewing-ring is positioned on the apex of the heart in a ‘no-touch-technique’ without heparin and the screw position is directed in parallel to the skin incision. Afterwards the ring is implanted using Teflon pledgeted sutures along with a 4-0 running Prolene suture. After sewing the HVAD-ring the ring along with the sutures are secured with biological glues such as Purastat glue, fibrin glue or even synthetic glues like BioGlue. Then, after assurance of 100% hemostasis at the LV apex, injection of i.v. heparin is followed by waiting for an appropriate activated clotting time (ACT). Cardiopulmonary bypass is then performed with arterial cannulation of the distal aorta ascendens and venous cannulation of the femoral vein or 2-stage cannulation of the RA, if there is enough space. After careful inspection of the left ventricular cavity to harvest potential thrombus formation or intraventricular trabecularization which might induce inflow disturbances, the HVAD pump is inserted through the HVAD ring, over the apex cordis, onto the left ventricle and the outflow graft is used to de-air and vent the left ventricle using the extracorporeal circulation (ECC)-succer. Afterwards, the ligature used to fasten the outflow vascular graft is clamped to the forceps and tunneled to the upper sternotomy site intra-pericardially. A side clamp is then set to the anterior section of the ascending aorta. An angled cut is made on the outflow graft and aortic anastomosis is implemented at the aorta ascendens with continuous 4-0 Prolene sutures through the mini-sternotomy. The driveline is set first in an umbilical direction in the rectus muscle sheath and then subcutaneously to the right or left upper abdominal quadrant. This approach has been shown to increase the subcutaneous driveline course, leading to lower risks of driveline infections (17).
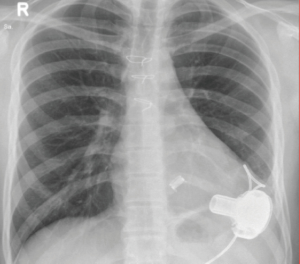
After the LVAD is de-aired and the absence of bubbles in the left ventricle is confirmed by transesophageal echocardiography, the pump is switched on in situ. The pump speed should be incrementally amplified while weaning the patient from the cardiopulmonary bypass with stable vital signs, no RV-failure signs and adequate AV-opening. An average pump flow-rate of 4.5 to 5±1 liters is typically achieved when the left pump is operating at 2,700±200 rpm. After closure of the two small wounds, the patient is relocated to the ICU where the first X-ray is performed (Figure 2).
Discussion and outlook
Conventional LVAD implantation is synonymous with large incisions, high complication rates, and overall poor outcomes, but the establishment of MIS procedures is revolutionizing the entire field (18). MIS for LVAD is not only technically feasible but also becoming the standard procedure of choice in VAD centres worldwide. Rapid development and miniaturization of next-generation devices, have significantly improved outcomes (2,19-21). The novel HVAD pump design is well suited for an MIS approach because of its small size. MIS-HVAD implantation by upper J hemisternotomy combined with anterolateral mini-thoracotomy entails several benefits (Table 1). This procedure is also feasible for other LVADs such as the HeartMate 3 (22).

Full table
Firstly, MIS procedures are associated with smaller incisions, less pain and bleeding, reduced reliance on transfusion products and anticoagulation drugs, shorter hospital stays and ultimately results in lower treatment costs (2,11,12). Secondly, the reduced incision sizes due to the hemisternotomy enable targeted access to the areas required for cannulation and the anterolateral mini-thoracotomy exposes the apex cordis without the need for cardiac displacement, which is a prerequisite for midline sternotomy. As is already known, in severe heart failure patients have poor tolerance for any form of cardiac manipulation. Thirdly, access is ultimately required for only two thoracic regions: the apex of the heart and the ascending aorta. Among all of the less invasive LVAD implant techniques described in literature, those two thoracic regions are separated into isolated incisions and surgical tasks. Thus, two surgeons can simultaneously expose the apex and the ascending aorta, thereby lowering the surgical operation time with improved postoperative outcome. Fourthly, despite recommendations from other reports on HVAD positioning on the LV diaphragmatic surface, our team has demonstrated that the pump placement with our new MIS technique leads to further optimized and stabilized inflow cannula placement through the anterolateral approach to the apex cordis (8,16). This technique prevents inflow obstruction and endocardial contact, hence reducing the risks of arrhythmias. Finally, this approach also reduces risks of developing right heart failure. The pericardium stays mainly unopened, enabling additional stabilization of right ventricular function including avoidance of right ventricular dilation during surgery, especially when the pump is switched on upon implantation (2).
There are some existing limitations of this technique which include the following: (I) the minimized surgical approach can be more technically challenging than a complete sternotomy; (II) accurate assessment of the outflow graft length can be more difficult than the conventional procedure; (III) limited visual confirmation of cardiac contractile function, particularly of the right ventricle and (IV) concomitant procedures such as valve replacement are technically more challenging or infeasible using the MIS approach (e.g., concomitant CABG procedure). Additionally, as opposed to other cardiothoracic procedures (such as coronary revascularizations or valve surgery), MIS-LVAD implantation is a fairly recent development. There is a dearth of comparative studies presenting middle-term or long-term results. Therefore, such minimized VAD-implantations should only be performed at centres with highly skilled cardiac surgeons with experience in other types of cardiac surgery who can diligently evaluate surgical results to enable the optimal quality of LVAD implantations.
In conclusion, HVAD implantation with this MIS approach is not only technically feasible but also associated with several advantages and will become the gold standard in the near future.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants of Medtronic. Other authors have no conflicts of interest to declare.
References
- Weir RAP, McMurray JJV, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol 2006;97:13F-25F. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2009;2:262-71. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Chen FY. Letter by Schmitto et al regarding article "Large animal models of heart failure: a critical link in the translation of basic science to clinical practice". Circ Heart Fail 2010;3:e3; author reply e4.
- Feldmann C, Chatterjee A, Haverich A, et al. Left ventricular assist devices - a state of the art review. Adv Exp Med Biol 2018;1067:287-94. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. HeartWare left ventricular assist device for the treatment of advanced heart failure. Future Cardiol 2016;12:17-26. [Crossref] [PubMed]
- Strueber M, Meyer AL, Feussner M, et al. A minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J Heart Lung Transplant 2014;33:851-6. [Crossref] [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [Crossref] [PubMed]
- Sileshi B, Haglund NA, Davis ME, et al. In-hospital outcomes of a minimally invasive off-pump left thoracotomy approach using a centrifugal continuous-flow left ventricular assist device. J Heart Lung Transplant 2015;34:107-12. [Crossref] [PubMed]
- Wu L, Weng YG, Dong NG, et al. Outcomes of HeartWare Ventricular Assist System support in 141 patients: a single-centre experience. Eur J Cardiothorac Surg 2013;44:139-45. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Gregoric ID, La Francesca S, Myers T, et al. A less invasive approach to axial flow pump insertion. J Heart Lung Transplant 2008;27:423-6. [Crossref] [PubMed]
- Anyanwu AC, Fischer GW, Plotkina I, et al. Off-pump implant of the Jarvik 2000 ventricular assist device through median sternotomy. Ann Thorac Surg 2007;84:1405-7. [Crossref] [PubMed]
- Slaughter MS. Implantation of the HeartWare left ventricular assist device. Semin Thorac Cardiovasc Surg 2011;23:245-7. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Hanke JS, et al. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif Organs 2014;38:507-10. [Crossref] [PubMed]
- Fleissner F, Avsar M, Malehsa D, et al. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices. Artif Organs 2013;37:102-7. [Crossref] [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Cvitkovic T, et al. First results of HeartWare left ventricular assist device implantation with tunnelling of the outflow graft through the transverse sinus. Interact Cardiovasc Thorac Surg 2017;25:503-8. [Crossref] [PubMed]
- Schmitto JD, Ortmann P, Akdis M, et al. Miniaturized HIA microdiagonal pump as left ventricular assist device in a sheep model. ASAIO J 2008;54:233-6. [Crossref] [PubMed]
- Gregoric ID, Bruckner BA, Jacob L, et al. Clinical experience with sternotomy versus subcostal approach for exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Ann Thorac Surg 2008;85:1646-9. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]