Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: a population-based study
Introduction
In 2015, the World Health Organization (WHO) reclassified a group of lung tumors (1). This new classification system divided adenocarcinomas into two categories of non-mucinous adenocarcinomas and adenocarcinoma variants, respectively. Nonmucinous and mucinous subtypes are the accepted types of preinvasive lesions (i.e., adenocarcinoma in situ) (1). Though debate remains, mucinous bronchoalveolar adenocarcinoma has been reclassified as invasive mucinous adenocarcinoma (IMA) (2,3). The classification of specific variants is based on the tumor’s pathological morphology, immunoprofile, and genetic and clinical characteristics. According to the new WHO classification system, mucinous adenocarcinoma and adenocarcinoma with mucin production are distinguished based on the presence of goblet or columnar cells in mucinous adenocarcinomas. Tumors previously designated as mucinous adenocarcinomas (ICD-O code 8480/3) are referred to as “colloid adenocarcinomas”, and also been reclassified as an adenocarcinoma variant (1).
When performing surgery, a mucinous adenocarcinoma can be easily identified by the presence of abundant mucin; however, its histological diagnosis can be mucinous adenocarcinoma, adenocarcinoma with mucin production, or colloid adenocarcinoma. Because mucinous adenocarcinoma is not a common subtype of lung tumors, its reported clinical outcomes have varied, and the terminologies used in study reports tend to be very heterogeneous. Because both mucin-producing adenocarcinoma and mucinous adenocarcinoma are frequently used together in the same report, it has been difficult to obtain a clear understanding of the disease entity (4-8).
The mucinous type of adenocarcinoma comprises between 2% and 10% of all lung tumors, and a large amount of data is required for a proper analysis. The results of previous studies of IMA have been based on relatively patient populations; therefore, the prognosis for patients with IMA remains controversial (6,7,9-11). The Surveillance, Epidemiology, and End Results (SEER) program sponsored by the National Cancer Institute in the United States has created a database that includes epidemiological, pathological, and survival information for all cancer cases reported in 18 regions of the United States since 1972.
The objective of our study was to assess the clinical presentation of IMA and then compare it with that of nonmucinous adenocarcinoma (NMA). We also compared the cancer-specific survival times in a matched cohort of surgically resected adenocarcinoma patients.
Methods
Patient selection
SEER*Stat 8.3.4 software was used to extract data from the SEER database. Patients were selected using the ‘SEER site Recode’ variable and the phrase “Lung and Bronchus”. Patients were also selected using the ‘Site and Morphology Behavior recode for analysis’ and the term “Malignant”. The international classification of disease for oncology-3 was used to restrict the pathology types to (8253/3), and NMA (ICD-O-3 codes 8140/3, 8250/3, 8252/3, 8253/3, 8254/3, 8255/4, 8551/3) (1). The NMA group included adenocarcinoma (8140/3), lepidic adenocarcinoma (8250/3), nonmucinous bronchioloalveolar carcinoma (8252/3), adenocarcinoma with mixed subtype (8255/3), papillary adenocarcinoma (8260/3), and acinar cell adenocarcinoma (8551/3). Other mucin-producing adenocarcinoma variants (i.e., mucin-producing adenocarcinoma, colloid adenocarcinoma, and a rare form of adenocarcinoma) were excluded from this study to avoid confusion.
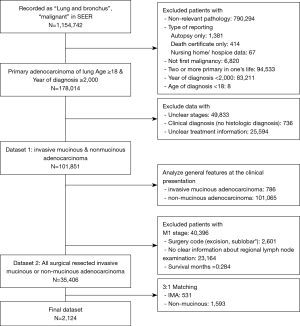
Next, the dataset was restricted to first malignancy, and the single primary malignancy in the patient’s life. The type of reporting source was restricted to other than an autopsy report or death certificate only cases. The process used for data cleansing is summarized in Figure 1. Two datasets were created, the first one (dataset 1) was used for describing the general features of the IMAs at the time of its clinical presentation. The second dataset (dataset 2) was used to assess the effect of specific histological characteristics on clinical outcomes after surgery was performed as the first course of treatment. Also, inclusion of a surgical cohort made the diagnosis of IMA more accurate, because its definitive diagnosis by transbronchial biopsy prior to surgery is frequently challenging.
Additional patient characteristics extracted from the dataset included basic demographic data, follow-up data, first course of treatment variables (radiation, chemotherapy, and surgery), TNM stages, pathologic characteristics of the tumor [size, location, and additional solitary pulmonary nodules (SPNs)], and cause of patient death.
Statistical analysis
The primary endpoint of this study was lung cancer specific survival (LCSS), as measured in months. Patients who were alive at the last follow-up date in the SEER database were right-censored at this date in the survival analysis.
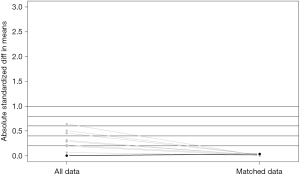
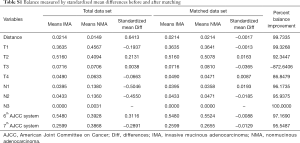
Characteristics were summarized using standardized statistical parameters, including the mean ± SD or median value for continuous variables, and frequencies and percentages for categorical variables. Distributions for continuous variables were analyzed using Student’s t-test or the Mann-Whitney U-test depending on the result of the Shapiro-Wilk test of normality. Categorical variables were compared using the chi-square test or Fisher’s exact test. The TNM and AJCC systems used for staging (3rd, 6th, and 7th system) were also used to match the IMA and NMA groups to enhance their comparability by the automated matching program from R (12). After matching, the balance between the two groups was checked via standardized mean differences, and the values for all the variables were <0.10 (Figure S1) (13,14).
The Kaplan-Meier method was used to obtain LCSS data and a log-rank test was used to compare survival curves for the IMA and NMA groups. Multivariate Cox proportional hazards (PH) models were used to estimate the association between tumor histological characteristics and cancer-specific survival in the unmatched group, and stratified Cox PH models were used for that purpose in the matched groups. The Shoenfeld residuals test was used to test the assumption of proportionality of hazards. The protocol for this study was approved by the Institutional Review Board for Seoul St. Mary’s Hospital (approval no. KC18ZESI0277).
Results
General features of IMAs and NMAs at the time of diagnosis
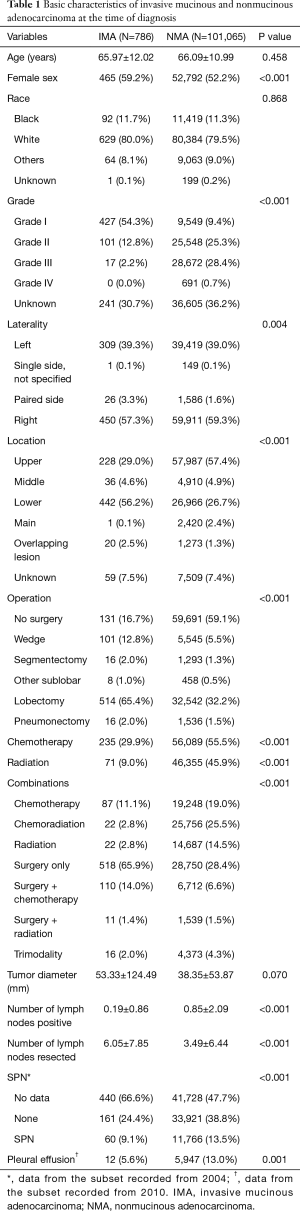
The general comparative features of the IMAs are shown in ‘dataset 1’ (Table 1). The incidence of IMAs among all lung cancer patients was 0.2% (1,783/1,153,742). While there were no differences in the age at diagnosis or racial distribution, there was a slight female predominance. Except when data for tumor differentiation was absent, most of the IMAs were well differentiated (Grade I), whereas the NMAs displayed various degrees of differentiation. Moreover, ~56.2% of the IMAs were located in the lower lobe of a lung. The IMAs tended to be larger than the NMAs at the time of diagnosis; however, this difference was not statistically significant. A subset of data recorded from 2010 onwards showed that pleural effusion was more common in the NMA group.
Full table
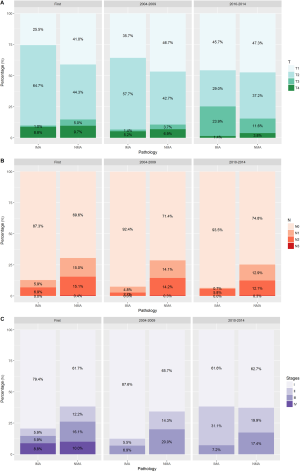
The AJCC staging system was used to create our dataset; therefore, we divided the entire cohort into three categories of ‘First [2000–2003]’, ‘Second [2004–2009]’, and ‘Third [2010–2014]’ periods according to the edition of the system used. Although there were several changes in the staging system, a comparison within the same period revealed that prior to and throughout 2009 the IMAs included more T2 stage tumors and fewer T1 stage tumors that did the NMAs. From 2010 onwards, the IMAs and NMAs had similar percentages of T1 and T2 tumors and greater percentages of T3 tumors (P<0.001, Figure 2). Regarding clinical and pathological N staging, most of the IMAs were N0 stage throughout the three periods. Approximately 20% of the IMA cases and 40% of the NMA cases had a distant metastasis at the time of diagnosis. Our dataset showed that no M1 cases of IMA or NMA were diagnosed prior to 2003. The most common first line therapy was surgery alone, and the most common type of surgery performed for cases of IMA was lobectomy.
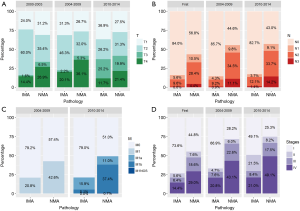
Clinical features of IMAs and NMAs in the surgically resected group
To assess the effect of tumor histology on the prognosis of patients who received surgical treatment, we limited our dataset to the M0 stage and applied a surgery code of at least ‘wedge’ or more. Moreover, we excluded data for cases in which unclear tumor stages were reported and no definite information concerning the number of regional lymph node examinations was provided. Additionally, we limited our dataset to include patients with an observed survival time of ≥1 month, for the purpose of excluding the statistical effect of immediate postoperative mortality (Figure 1).
Our ‘dataset 2’ had 531 patients with an IMA and 34,875 patients with a NMA, for an overall incidence rate of 1.5%. There were no differences in the distributions of patient age, race, and insurance status at the time of diagnosis (Table 2). Patients in the IMA group had tumors that were more differentiated (either Grade I or II), showed a lower lobe location preference, and had larger diameters when compared with patients in the NMA group. The IMA patients also had more regional lymph nodes surgically examined than did the NMA patients. There were no significant differences in the laterality of the tumor’s location, and the stage distribution of the dataset 2 was similar to the dataset 1 (Figure S1). The data specific for SPNs and tumors that showed pleural invasion were available for patients entered into the SEER database after 2004 and 2010, respectively. This data subset showed that the occurrence of SPNs was not significantly different in the two groups (IMA and NMA), while pleural involvement occurred less frequently with IMAs than NMAs.
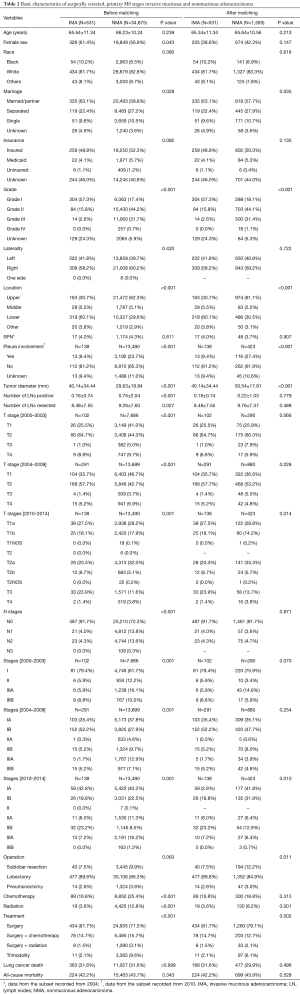
Full table
The dataset showed that the most common first-line treatment was surgery alone (81.7% of IMA cases and 71.5% of NMA cases). Approximately 15% of patients received chemotherapy as neoadjuvant or adjuvant therapy, and ~1.5% of IMA patients and 3.1% of all patients received both surgery and radiation. Approximately 2.1% of IMA patients and 9.6% of NMA patients received trimodality therapy as their first-line treatment. Among the patients who received surgery in both groups, >85% received a lobectomy or bilobectomy. Only ~10% of all surgical patients both groups received a pneumonectomy, segmentectomy or wedge resection as first-line therapy.
Effect of an IMA on survival after surgery: a matched dataset analysis
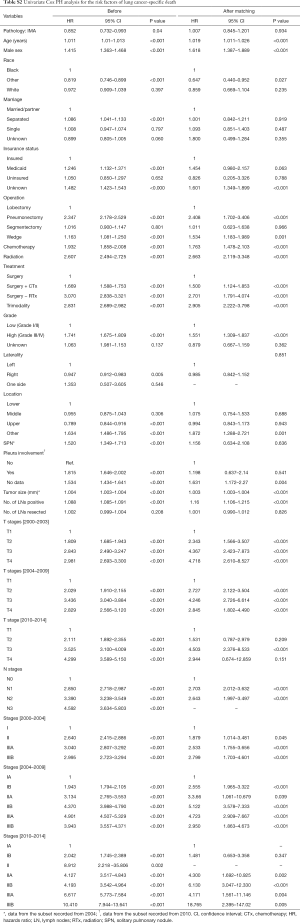
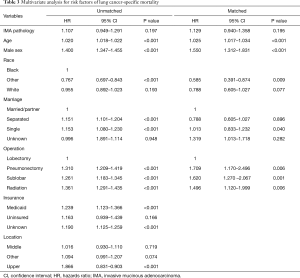
After matching via T stage, N stage, and the AJCC system (3rd, 6th, 7th systems), balance testing performed by using changes in absolute standardized mean values and mean differences gave results that were all <0.10, indicating well-balanced matching (Figure S2, Table S1). Based on results of a univariate analysis performed after matching, we then constructed a multivariate stratified Cox PH model. A univariate analysis performed with the matched dataset revealed that patient age at diagnosis, male sex, a higher tumor grade (Grade III or IV), and higher T and N stages were all associated with LCSS (Table S2). Tumor laterality (P=0.851), presence of a SPN (P=0.636), pleural involvement (P=0.541), and the total numbers of regional lymph nodes examined (P=0.826) were not associated with LCSS.
Full table
Full table
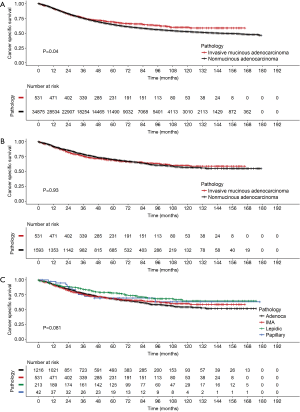
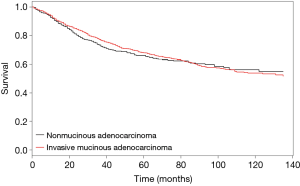
Because the tumors were matched by T, N, and AJCC system variables, we performed a Cox PH analysis that was stratified by those three variables. The results indicated that the pathological type of the tumor (either IMA or NMA) was not associated with LCSS when other confounding variables such as race, marital status, age, and sex were controlled (Table 3). Before matching, the IMA patients showed a longer mean survival time than the NMA patients (59.3±41.9 months for IMA patients vs. 49.8±42.0 months for NMA patients, P=0.04) (Figure 3A). However, after matching, the effect of histologic type on cancer-specific survival was no long observed (Figure 3B). The mean number of survival months in the matched dataset was 59.3±41.9 months for IMA patients and 56.2±41.8 months for NMA patients (P=0.93). Though statistically insignificant, the survival curve was located between the lepidic adenocarcinoma and general other types of adenocarcinoma (Figure 3C). An age and sex adjusted Cox PH model also showed no statistically significant differences in the matched dataset (Figure 4).
Full table
Discussion
A primary pulmonary IMA is distinguished from other tumors by its abundant intracytoplasmic mucin produced by goblet or other columnar cells. IMAs are rare, and account for <2–10% of all lung adenocarcinomas (15). ICD-O-3 histology code 8253/3 denotes an IMA; previously termed a ‘mucinous bronchioloalveolar carcinoma’ (16). We specifically wanted to focus on this entity because there remain some issues concerning tumor classifications. First, because NMAs are known to produce mucin, distinguishing an NMA from an IMA can be difficult. Second, the definition and quantification of mucin production have not been well-defined. Third, other types of mucin-producing adenocarcinomas have been identified, including colloid adenocarcinoma, cystadenocarcinoma, and signet ring cell carcinoma (3).
Due to the low incidence of IMAs, a large data source is necessary to overcome statistical power issues and accurately identify general features of the disease. The SEER database was well suited for this purpose, and revealed that the incidence of IMA among resected adenocarcinomas was 1.5%. A further analysis of the data allowed us to identify several features of IMAs. First, IMAs in the lung showed a strong tendency for a lower lobe location. At the time of diagnosis, 56.2% of IMAs were located in the lower lobe, while NMAs were predominantly located in an upper lobe. Second, the incidence of bilateral location was 3.3% in the IMA group, which was 2-fold higher than that in the NMA group. These findings are consistent with reports stating that IMAs have a tendency for bilateral lung involvement and lower lobe predominance (16-18). However, due to a lack of information, we could not confirm some other known features of IMAs, such as their multicentricity and multilobar location. Data for SPNs only become available starting in 2004, and was mostly absent for the period that we studied. Therefore, we cannot draw any conclusions concerning the multicentricity and preferred locations of SPNs. Finally, while IMAs are commonly thought to be diagnosed at an advanced inoperable stage, we found that at the time of diagnosis, ~70% of IMAs were either stage I or II.
Patients with an IMA are known to have poor overall survival and progression-free survival times when compared to patients with other lung adenocarcinoma subtypes. They are also thought to present at an advanced stage of their disease which cannot be treated by surgery (2,6,19). However, a very recent study by Shim et al. demonstrated that the overall survival time of IMA patients is comparable to that of patients with intermediate grade NMA (10). Furthermore, a Japanese study of 440 patients with an adenocarcinoma revealed that the disease-free survival times of IMA patients were intermediate between the low grade and high grade adenocarcinoma groups (20). Finally, a study by Warth et al. showed that IMA patients had a better prognosis than most adenocarcinoma patients (7). Though statistically insignificant, the IMA patients in our study cohort also had survival curves that were intermediate between those for patients with lepidic adenocarcinoma and other adenocarcinomas (Figure 3C). A survival analysis of the matched dataset showed that the rate of 5-year LCSS was 69.3% with a median LCSS time of 51.0 months in the IMA group, and 70.1% with a median LCSS time of 49.0 months in the NMA group. Lee et al reported a 5-year disease-free survival rate of 79% and a median disease-free survival time of 46.2 months, which is comparable to the findings in this study (10). After matching was performed, univariate and multivariate stratified Cox PH analyses revealed that LCSS times were not affected by tumor histologic characteristics. Rather, after adjusting for confounding variables, the patients who required radiation (HR 1.5, P=0.01), pneumonectomy (HR 1.71, P=0.01) or sublobar resection (HR 1.620, P<0.001) had shorter LCSS times.
Data pertaining to nodal status in our study cohort revealed that at the time of diagnosis, >80% of IMA cases were at the N0 stage throughout the entire study period (84.0% for years 2000–2003, 85.7% for years 2004–2009, and 82.7% for years 2010–2014), which was double the percentage of N0 stage cases in the NMA group. This finding is in agreement with previous small studies that reported lower rates of nodal metastasis and fewer lymphatic invasions (9,10,21). Although the association between tumor genotype and histology is not absolute, the lower N stages might be related to the characteristic molecular profile of IMAs. For example, KRAS mutations have been observed in 60–76% of IMAs, while EGFR mutations are absent in IMAs (21,22). Kakegawa et al. suggested that KRAS mutant tumors might grow faster than tumors without KRAS mutations which might explain why in our study, the IMA group had larger tumor sizes than the NMA group (23). The effect of genotype on tumor behavior and clinical outcomes needs to be verified in future large-scale studies.
This study has several limitations. First, there was no data regarding disease-free survival or recurrence in the SEER database. However, as the SEER database is controlled by the National Cancer Institute, and the numbers of deaths caused by specific cancers are available, the rates and time periods of disease-free survival can be inferred from the rates and times periods of cancer-specific survival. Second, the SEER database lacks patient comorbidity profiles. This lack of comorbidity information is partially why we chose the surgically treated dataset for our main analysis, as the physical condition of patients eligible for surgery would be less heterogenous than that of the entire dataset. Third, because we could not review the pathology findings for individual tumor specimens, our grouping was based on the ICD-O-3 codes of the enrolled dataset. While issues concerning accuracy and inaccuracy might arise, the SEER registrar reviews the data on a continuous basis. Furthermore, we strictly limited the ratio of NMA cases to IMA cases to reduce any possible bias. Moreover, the sample sizes used in this study are the largest to have been reported. Although our study had neither a prospective nor randomized design, it was multi-institutional, and reflects real-world clinical presentations, practices, and disease outcomes. This fact compensates for its limitations and allows us to establish a tentative relationship between tumor pathological characteristics and LCSS.
In conclusion, the histologic subtype of IMA did not affect LCSS in surgically resected, M0 stage, primary lung adenocarcinoma patients. In this group of patients, those who needed radiation, chemotherapy or an extensive surgery such as pneumonectomy had shorter LCSS times.
Acknowledgements
We would like to give special thanks to the biostatistical consulting team of our institute.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol for this study was approved by the Institutional Review Board for Seoul St. Mary’s Hospital (approval no. KC18ZESI0277).
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Dacic S. Pros: the present classification of mucinous adenocarcinomas of the lung. Transl Lung Cancer Res 2017;6:230-3. [Crossref] [PubMed]
- Popper HH. Cons: the confusing mucinous adenocarcinoma classification. Transl Lung Cancer Res 2017;6:234-40. [Crossref] [PubMed]
- Liu Y, Zhang HL, Mei JZ, et al. Primary mucinous adenocarcinoma of the lung: A case report and review of the literature. Oncol Lett 2017;14:3701-4. [Crossref] [PubMed]
- Shim HS, Kenudson M, Zheng Z, et al. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 2015;10:1156-62. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Oka S., Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg 2010;33:89-93. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac oncol 2011;6:1496-504. [Crossref] [PubMed]
- Lee HY, Cha MJ, Lee KS, et al. Prognosis in Resected Invasive Mucinous Adenocarcinomas of the Lung: Related Factors and Comparison with Resected Nonmucinous Adenocarcinomas. J Thorac Oncol 2016;11:1064-73. [Crossref] [PubMed]
- Boland JM, Maleszewski JJ, Wampfler JA, et al. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma-a clinicopathological and molecular genetic study with survival analysis. Hum Pathol 2018;71:8-19. [Crossref] [PubMed]
- Ho D, Imai K, King G, et al. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. 2011;42:28. Available online: http://jstatsoft.org/v042/i08
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician 1985;39:33-8.
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Tang ER, Schreiner AM, Pua BB. Advances in lung adenocarcinoma classification: a summary of the new international multidisciplinary classification system (IASLC/ATS/ERS). J Thorac Dis 2014;6:S489-S501. [PubMed]
- Manning JT Jr, Spjut HJ, Tschen JA. Bronchioloalveolar carcinoma: The significance of two histopathologic types. Cancer 1984;54:525-34. [Crossref] [PubMed]
- Cha YJ, Kim HR, Lee HJ, et al. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer 2016;102:82-8. [Crossref] [PubMed]
- Wislez M, Antoine M, Baudrin L, et al. Non-mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer 2010;68:185-91. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Kadota K, Yeh YC, D’Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol 2014;38:1118-27. [Crossref] [PubMed]
- Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn 2007;9:320-6. [Crossref] [PubMed]
- Kakegawa S, Shimizu K, Sugano M, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer 2011;117:4257-66. [Crossref] [PubMed]