Genetic and pharmacological inhibition of eIF4E effectively targets esophageal cancer cells and augments 5-FU’s efficacy
Introduction
Esophageal cancer is one of the most common forms of cancer worldwide, and its prevalence has increased in recent years. Despite substantial advances in diagnostics and therapeutics, the clinical management of esophageal cancer is still challenging, with a 5-year survival rate of less than 25% (1). The molecular mechanisms leading to esophageal cancer progression are complex and not well known. A clear understanding of the molecular pathogenesis of esophageal cancer is required for the development of novel targeted therapies.
Eukaryotic translation initiation factor 4E (eIF4E) is a eukaryotic translation initiation factor. One of its functions involves guiding ribosomes to the 5' cap structure of mRNAs and thus is critical in cap-dependent translation (2,3). Importantly, eIF4E phosphorylation does not alter global translation rates, and mice developed normally in the absence of phosphorylated eIF4E (4). eIF4E also preferentially promotes the translation of carcinogenesis-associated mRNAs, such as c-Myc and vascular endothelial growth factor (VEGF), which contributes to the aberrant proliferation and survival of cancer cells (3,5-7). Aberrant eIF4E activation and overexpression is common and correlates with a poorer prognosis in various cancers, including leukemia, ovarian cancer, and hepatocellular carcinoma (8-10). In addition, eIF4E has been shown to play a critical role in tumor transformation, progression, and metastasis, and its activity is upregulated in cancer cells in response to chemo- or radiotherapy (11-14). Therapeutic suppression of eIF4E reduces tumor cell growth without toxicity (15). Ribavirin is an FDA-approved antiviral drug with documented activity against eIF4E and is effective in targeting various types of cancer (3,16,17). In patients with esophageal cancer, eIF4E expression is increased in the cancerous compared to noncancerous esophageal tissues and its higher expression is correlated with the more advanced stages of the disease (18). However, the possible roles of eIF4E in esophageal cancer are largely unknown.
In this study, we compared eIF4E expression in esophageal cancer cell lines and normal esophageal epithelial cell lines, and investigated the roles of eIF4E in esophageal cancer. We demonstrate that eIF4E mRNA and protein levels are elevated in esophageal cancer cells. eIF4E inhibition significantly reduces esophageal cancer cell growth and survival, and sensitizes the cells to 5-flurouracil (5-FU) treatment.
Methods
Cells and reagents
Four human esophageal carcinoma cell lines were obtained from Sigma (USA) and cultured in RPMI 1640 medium supplemented with 2 mM glutamine and 10% fetal bovine serum. Immortalized normal human esophageal epithelial cell lines NE2-hTERT (Abm, USA) and HTA-1A (ATCC, USA) were cultured in a 1:1 ratio of Defined Keratinocyte-SFM (DKSFM, Gibco, #10744-019) and Epilife medium (Cascade Biologics, #M-EPI-500-CA) with Epilife Defined Growth Supplement (Cascade Biologics, #S-012-5). Ribavirin (Kemprotec Ltd.) and 5-FU (Sigma, USA) were reconstituted in water and DMSO, respectively, and stored at −20 °C in aliquots.
In vitro assays for cell proliferation and viability
Cell proliferation was assessed by using the CellTiter® One Solution Cell Proliferation Assay (Promega, USA). Cell viability was determined via flow cytometry using Annexin V-FITC/7-AAD (BD Pharmingen, US) staining. Annexin V(-)/7-AAD(-) were considered as viable cells. The specific conditions for each experiment are described in the figure legends.
Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot (WB) analyses
Four percent SDS was used to lyse cells and the total protein content was quantified by bicinchoninic acid protein assay kit (Thermo Scientific, USA). Proteins were resolved using denaturing SDS-PAGE and analyzed by WB using antibodies against phosphorylated and total eIF4E, Akt, mTOR, β-catenin, VEGF, c-Myc and β-actin (Santa Cruz Biotechnology, USA). Immunoblots shown in the accompanying figures are representative of three independent experiments.
Transfection
OE33 stable cell lines overexpressing wildtype eIF4E and its mutant forms (S209A and S209D) were generated by transducing cells with retroviral MSCV-internal ribosome entry site (IRES) constructs containing eIF4E WT, S209D, and S209A, as previously described (19). Specific knockdown of eIF4E was achieved by transfecting cells with eIF4E siRNA (100 nM) with Lipofectamine TM 2000 and OptiMEM (Invitrogen, US) as per the manufacturer’s protocol. eIF4E siRNA and plasmids were obtained from Dr. Cui Sha’s laboratory (20) as kind gifts.
Real-time PCR
Total RNA and cDNA preparation were carried out using TRIzol (Invitrogen, USA) and iScript cDNA Synthesis Kit (Bio-rad, CA, USA), respectively, as per the manufacturer’s protocol. The human eIF4E RNA was detected by quantitative real-time PCR using the prepared cDNA as a template. The eIF4E primer sequences were: forward primer, 5'-TGGCGACTG TCGAACCG-3' and reverse primer, 5'-AGATTCCGTTTTCTCCTCTTCTGTAG-3'.
Esophageal carcinoma xenograft and immunohistochemistry in SCID mice
The animal experiments were approved by the Institutional Animal Care and Use Committee of Hubei Cancer Hospital. Athymic mice at 6 weeks-old were purchased from Biocytogen Inc, China. OC33 cells (5×106) were subcutaneously injected into the right flank of the mouse. When the tumor volume (calculated as length × length × width/2) reached ~100 mm3, the mice were randomized into four groups receiving vehicle control, intraperitoneal 0.5 mg/kg 5-FU three times a week, oral 30 mg/kg ribavirin once daily, or a combination of both for 21 days. After treatment, mice were euthanized and tumors were isolated. The tumors were fixed in 4% paraformaldehyde (PFA, Sigma Aldrich, USA) and sectioned. Apoptotic tumor cells were assessed using the TUNEL Assay Kit (R&D System, USA) and proliferating cells were labeled by a PCNA antibody as per manufacturer’s protocol. The nuclei were counterstained with haematoxylin (Sigma).
Statistical analyses
Data are expressed as mean ± standard deviation (SD). Statistical analyses of the differences between two groups were performed using the one-way analysis of variance (ANOVA) and subsequently by the unpaired Student’s t-test. P values <0.05 were considered statistically significant.
Results
eIF4E is involved in growth, survival, and chemoresistance of esophageal cancer cells
eIF4E levels are elevated in cancerous esophageal tissues as compared to normal adjacent tissues (18). Similar to that observed in patient samples, we show that the level of eIF4E mRNA is significantly higher in a panel of esophageal cancer cell lines (OE33, ESO26, FLO-1, and KYAE-1) than immortalized normal esophageal epithelial cell lines (NE2-hTERT and HTA-1A) (Figure 1A). The selected esophageal cancer cell lines have been verified and represent human esophageal cancer cells (21). Furthermore, we show here that eIF4E protein levels are significantly higher in esophageal cancer cells than in their normal counterparts (Figure 1B). Our results are consistent with previous findings and demonstrate that esophageal cancer cell lines are a representative in vitro model of esophageal cancer with respect to their eIF4E expression.
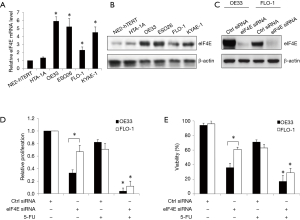
We next investigated how eIF4E loss-of-function would affect the biological activities of esophageal cancer cells. In the eIF4E functional studies, we selected OE33 and FLO-1 cell lines as they represent the cells with the highest and lowest expression levels of eIF4E, respectively (Figure 1A,B). We demonstrated the remarkable reduction of eIF4E protein level in esophageal cancer cells transfected with specific eIF4E siRNA but not control siRNA (Figure 1C). eIF4E depletion significantly inhibited the growth and survival of esophageal cancer cells (Figure 1D,E). Notably, OE33 cells, which have high eIF4E levels, are significantly more sensitive to eIF4E depletion as compared to FLO-1 cells, which have low eIF4E levels (Figure 1D,E). In addition, eIF4E depletion significantly augments the inhibitory effects of 5-FU (a commonly used chemotherapeutic agent for esophageal cancer) on the growth and survival of esophageal cancer cells (Figure 1D,E).
Ribavirin mimics the effects of eIF4E depletion in esophageal cancer cells.
We next inhibited eIF4E using pharmacological inhibitor ribavirin. We treated cells with ribavirin at 10, 25 and 50 µM which are effective concentrations demonstrated in other cancers, such as retinoblastoma and glioblastoma (17,20). We found that ribavirin significantly inhibited proliferation and decreased viability of esophageal cancer cells in a dose-dependent manner (Figure 2A,B). Similar to eIF4E depletion, OE33 cells were more sensitive to ribavirin treatment than FLO-1 (Figure 2A,B). We next performed combination studies using ribavirin and standard chemotherapeutic drug 5-FU using a concentration that was sublethal as a single drug. We found that combination of ribavirin and 5-FU was significantly more effective in targeting esophageal cancer cells compared to ribavirin or 5-FU alone (Figure 2C,D). Almost full inhibition of growth and survival of cells were achieved by the combination.

Ribavirin acts on esophageal cancer cells through inhibiting Akt/mTOR/eIF4E
To confirm that the molecular mechanism of ribavirin’s action on esophageal cancer cells was through eIF4E suppression, we analyzed the levels of active eIF4E and its translationally regulated proteins. We observed a dose-dependent decrease on p-eIF4E and its regulated protein c-Myc and VEGF levels in OE33 cells (Figure 3A). We also observed the decreased levels on p-Akt, p-mTOR and but not p-β-catenin (Figure 3A), indicating the specific inhibition of ribavirin on Akt/mTOR/eIF4E signaling pathway in esophageal cancer. Overexpressing wildtype and phosphor-mimetic form (S209D) of eIF4E (22) but not nonphosphorylatable form (S209A) significantly abolished the inhibitory effects of ribavirin on growth and survival (Figure 3B,C). This clearly demonstrated that ribavirin inhibited eIF4E in esophageal cancer, and this was dependent on the phosphorylation at S209.

Ribavirin augments 5-FU’s inhibitory effects in esophageal cancer in vivo
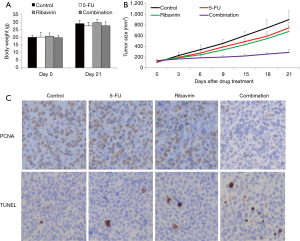
We further investigated the effects of eIF4E inhibition in vivo and the translational potential of ribavirin in the well-established xenograft esophageal cancer mouse model of athymic mice and OE33 cells (23). Mice tolerated 30 mg/kg ribavirin and 0.5 mg/kg 5-FU very well, as no significant weight loss or abnormal appearance was registered (Figure 4A). We observed that ribavirin or 5-FU as a single drug modestly inhibited tumor growth, but the tumors continued to increase in size (Figure 4B). However, when the drugs were used in combination, we observed tumor growth arrest throughout the duration of treatment. To investigate whether the growth inhibition was due to decreased cell growth and increased cell death, as observed in the cell culture study, we performed PCNA and TUNEL staining on tumor tissues. Consistent with the data obtained from the cell culture system, we observed less proliferation and more apoptotic cells in the combination group (Figure 4C), which confirmed the enhanced efficacy of using 5-FU and ribavirin for inhibiting esophageal cancer cell proliferation and inducing apoptosis.
Discussion
Neoadjuvant chemotherapy using 5-FU-based regimes is used for patients with advanced esophageal cancer (24). However, patients easily develop resistance to chemotherapy and usually relapse within a year (25). Furthermore, the mechanisms underlying the persistence of esophageal cancer cells in patients receiving chemotherapy are not well understood. Thus, a better understanding of the specific drivers of esophageal cancer is important to the development of more effective and less toxic therapeutic strategies to overcome this resistance. eIF4E is aberrantly activated in a variety of cancers and preferentially contributes to tumor progression and protects tumor cells from chemotherapy (11-14). eIF4E has been shown to be upregulated in esophageal cancer tissues and its expression is associated with poor prognosis (18). However, little is known on how eIF4E facilitates esophageal cancer growth and chemoresistance. In this work, we are the first to provide preclinical evidence to demonstrate that eIF4E is critically involved in the growth, survival, and chemotherapeutic resistance of esophageal cancer cell lines and xenograft mouse model tumors.
Our study demonstrated that the eIF4E mRNA and protein levels were higher in a panel of esophageal cancer cell lines as compared to immortalized normal epithelial cell lines (Figure 1A,B), and this was consistent with that observed in human esophageal cancer tissues and adjacent normal tissues (18). This directly supports the work by Salehi et al. (18) and indicates that esophageal cancer cell lines used in our study are in vitro models that well represent the in vivo disease. In addition, eIF4E expression level varies among the four esophageal cancer cell lines with the highest expression being present in OE33 and the lowest in FLO-1. This is also in agreement with Salehi and colleagues (18) who demonstrated that eIF4E level varies in esophageal cancer specimens. Our findings suggest that esophageal cancer patients with different eIF4E expression levels might respond differently to therapeutic eIF4E inhibition.
eIF4E inhibition using the pharmacological inhibitor ribavirin (26-28) and eIF4E knockdown using inhibitory RNA significantly suppresses the growth and survival of esophageal cancer cells, and augments 5-FU’s efficacy (Figures 1C,D,E,2). Notably, OE33 cells are more sensitive to eIF4E depletion but not 5-FU treatment than FLO-1 cells. The different sensitivity to eIF4E inhibition in esophageal cancer cell lines correlates well with their expression levels and dependence on eIF4E (Figure 1). The positive roles of eIF4E in growth, survival, and metastasis have been shown in various cancers, including renal cell carcinoma, retinoblastoma, and leukemia (16,20,29). Our work adds esophageal cancer onto the list of eIF4E-regulated cancers.
A key finding in this study is that we identified ribavirin as an eIF4E inhibitor, which is also an FDA-approved antiviral drug with a known pharmacological profile and is effective against esophageal cells at a clinically achievable dose (30). We further demonstrate that ribavirin acts on esophageal cancer cells through suppressing the Akt/mTOR/eIF4E pathway (Figure 3). The potent and selective anti-cancer activities of ribavirin have been demonstrated in various cancers and the translational potential of ribavirin in oropharyngeal squamous cell carcinoma and metastatic breast cancer are under investigation (3,31-34). Although ribavirin is an antiviral drug that prevents viral replication through inhibiting viral RNA synthesis and mRNA capping, its potent anti-cancer activities have been recently observed in various cancers, such as leukemia, retinoblastoma, renal cancer, and tongue squamous carcinoma (16,20,35,36). The mechanisms of action of ribavirin on cancer vary across the different types of cancers, which include eIF4E inhibition, mTOR signaling suppression, and the downregulation of the guanosine biosynthetic pathway (30,33,35,36). Notably, our work and many other studies (26,30,33) demonstrate that eIF4E inhibition is likely to be the predominant mechanism of action for ribavirin’s anti-cancer effects.
We further show that the combination of ribavirin and 5-FU at sublethal concentrations achieved almost complete inhibition in esophageal cancer in vitro, as well as in vivo, without significant toxicity (Figures 2,4). This demonstrates that ribavirin effectively sensitizes esophageal cancer cells to 5-FU, and is consistent with previous studies that indicate that ribavirin enhances the efficacy of other chemotherapeutic agents (31,32,36). We speculate that the enhanced efficacy of the combination of 5-FU and ribavirin is due to the dual inhibition of the Akt/mTOR/eIF4E pathway and DNA synthesis in esophageal cancer cells. Our results are consistent with that of Graff et al. (15), which showed that the suppression of eIF4E expression reduces tumor growth without toxicity and further demonstrates that eIF4E is a selective therapeutic target in cancer.
In conclusion, we used genetic and pharmacological methods in cell culture and xenograft models, coupled with mechanism studies to identify the critical involvement of eIF4E in esophageal cancer cell growth and survival. Given ribavirin’s known pharmacokinetics and toxicology in humans as an antiviral drug, our findings also suggest that ribavirin is an attractive candidate to sensitize esophageal cancer cell to chemotherapy.
Acknowledgements
This work was supported by a research grant provided by Ministry of Education.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18:1926-45. [Crossref] [PubMed]
- Kentsis A, Topisirovic I, Culjkovic B, et al. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A 2004;101:18105-10. [Crossref] [PubMed]
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, et al. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 2004;24:6539-49. [Crossref] [PubMed]
- Bhat M, Robichaud N, Hulea L, et al. Targeting the translation machinery in cancer. Nat Rev Drug Discov 2015;14:261-78. [Crossref] [PubMed]
- Mamane Y, Petroulakis E, Martineau Y, et al. Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS One 2007;2. [Crossref] [PubMed]
- Larsson O, Li S, Issaenko OA, et al. Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res 2007;67:6814-24. [Crossref] [PubMed]
- Liu S, Zha J, Lei M. Inhibiting ERK/Mnk/eIF4E broadly sensitizes ovarian cancer response to chemotherapy. Clin Transl Oncol 2018;20:374-81. [Crossref] [PubMed]
- Lim S, Saw TY, Zhang M, et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci U S A 2013;110:E2298-307. [Crossref] [PubMed]
- Jiang XM, Yu XN, Huang RZ, et al. Prognostic significance of eukaryotic initiation factor 4E in hepatocellular carcinoma. J Cancer Res Clin Oncol 2016;142:2309-17. [Crossref] [PubMed]
- Grzmil M, Seebacher J, Hess D, et al. Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy. Cell Signal 2016;28:1412-21. [Crossref] [PubMed]
- Xu H, Wang Z, Xu L, et al. Targeting the eIF4E/beta-catenin axis sensitizes cervical carcinoma squamous cells to chemotherapy. Am J Transl Res 2017;9:1203-12. [PubMed]
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature 1990;345:544-7. [Crossref] [PubMed]
- Zimmer SG, DeBenedetti A, Graff JR. Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Res 2000;20:1343-51. [PubMed]
- Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest 2007;117:2638-48. [Crossref] [PubMed]
- Cao J, Sun X, Zhang X, et al. Inhibition of eIF4E cooperates with chemotherapy and immunotherapy in renal cell carcinoma. Clin Transl Oncol 2018;20:761-7. [Crossref] [PubMed]
- Volpin F, Casaos J, Sesen J, et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene 2017;36:3037-47. [Crossref] [PubMed]
- Salehi Z, Mashayekhi F. Expression of the eukaryotic translation initiation factor 4E (eIF4E) and 4E-BP1 in esophageal cancer. Clin Biochem 2006;39:404-9. [Crossref] [PubMed]
- Kharas MG, Deane JA, Wong S, et al. Phosphoinositide 3-kinase signaling is essential for ABL oncogene-mediated transformation of B-lineage cells. Blood 2004;103:4268-75. [Crossref] [PubMed]
- Wang G, Li Z, Li Z, et al. Targeting eIF4E inhibits growth, survival and angiogenesis in retinoblastoma and enhances efficacy of chemotherapy. Biomed Pharmacother 2017;96:750-6. [Crossref] [PubMed]
- Boonstra JJ, van Marion R, Beer DG, et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst 2010;102:271-4. [Crossref] [PubMed]
- Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev 2007;21:3232-7. [Crossref] [PubMed]
- Melsens E, De Vlieghere E, Descamps B, et al. Improved xenograft efficiency of esophageal adenocarcinoma cell lines through in vivo selection. Oncol Rep 2017;38:71-81. [Crossref] [PubMed]
- Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 2013;61:330-5. [Crossref] [PubMed]
- Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg 2009;138:1309-17. [Crossref] [PubMed]
- Crotty S, Cameron C, Andino R. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J Mol Med (Berl) 2002;80:86-95. [Crossref] [PubMed]
- Assouline S, Culjkovic B, Cocolakis E, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood 2009;114:257-60. [Crossref] [PubMed]
- Loustaud-Ratti V, Debette-Gratien M, Jacques J, et al. Ribavirin: Past, present and future. World J Hepatol 2016;8:123-30. [Crossref] [PubMed]
- Kosciuczuk EM, Saleiro D, Platanias LC. Dual targeting of eIF4E by blocking MNK and mTOR pathways in leukemia. Cytokine 2017;89:116-21. [Crossref] [PubMed]
- Pettersson F, Yau C, Dobocan MC, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res 2011;17:2874-84. [Crossref] [PubMed]
- Shi F, Len Y, Gong Y, et al. Ribavirin Inhibits the Activity of mTOR/eIF4E, ERK/Mnk1/eIF4E Signaling Pathway and Synergizes with Tyrosine Kinase Inhibitor Imatinib to Impair Bcr-Abl Mediated Proliferation and Apoptosis in Ph+ Leukemia. PLoS One 2015;10. [Crossref] [PubMed]
- Kraljacic BC, Arguello M, Amri A, et al. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia 2011;25:1197-200. [Crossref] [PubMed]
- Tan K, Culjkovic B, Amri A, et al. Ribavirin targets eIF4E dependent Akt survival signaling. Biochem Biophys Res Commun 2008;375:341-5. [Crossref] [PubMed]
- Kentsis A, Volpon L, Topisirovic I, et al. Further evidence that ribavirin interacts with eIF4E. RNA 2005;11:1762-6. [Crossref] [PubMed]
- Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma 2010;51:1805-15. [Crossref] [PubMed]
- Dai D, Chen H, Tang J, et al. Inhibition of mTOR/eIF4E by anti-viral drug ribavirin effectively enhances the effects of paclitaxel in oral tongue squamous cell carcinoma. Biochem Biophys Res Commun 2017;482:1259-64. [Crossref] [PubMed]


