Lung ultrasound as a monitoring tool in lung transplantation in rodents: a feasibility study
Introduction
Orthotopic lung transplantation in rats has been developed as a model to study graft dysfunction (1) but effective tools for monitoring it (2) are not yet available. Micro-computer tomography, micro-magnetic resonance and micro-positron emission tomography have been proposed as mini invasive monitoring systems (3,4), though their use is limited by the costs. Moreover, these tests must be performed under deep sedation or general anesthesia (GA), hence the effects of hypnotic drugs on tidal volumes and inspiratory efforts could influence the obtained results.
Point-of-care ultrasound is a rapid, radiation free, non-invasive, repeatable and bedside feasible technique. It has recently emerged as a reliable tool in the management of several pathological conditions in critically ill human patients (5). Lung ultrasound (LUS) has been demonstrated to have an impact on the diagnosis and treatment of pneumonia (6), pulmonary edema (7), pleural effusion (8), pneumothorax (9), interstitial pathologies (10), Positive end-expiratory pressure titration (11) and dyspnea (12). An algorithm to predict weaning failure from mechanical ventilation has also been proposed considering the use of LUS combined with echocardiography and the sonographic study of the diaphragm (13).
The interactions between ultrasound and lung tissues with different densities create real images and also artifacts, which reveal information about pleura and parenchyma (14). The normal pattern includes the visualization of pleural line with the sliding of the visceral on the parietal pleura and the horizontal artifacts underneath, named A-lines. In conditions with increased lung parenchymal density, the partial penetration of ultrasounds into lung tissue generates vertical artifacts called B lines, and the more the density, the higher the number of B lines (15,16). Differences in density can depend on edema, atelectasis or consolidation, and when density becomes comparable to that of a parenchymal organ, ultrasound can pass through the tissues and produce a real image of the consolidated lung (17).
Considering rat as a model of left lung transplantation, a failure in graft function is hard to be recognized based only on clinical data because of the small size of the left lung related to the right lung. The total lung capacity is about 10 mL and this volume is distributed in five lobes on the right side and one on the left side (18). If a left graft failure occurs (due to acute rejection or atelectasis), the loss of function can be compensated with minimal impact on respiratory pattern and the diagnosis could be delayed. Circulating biomarkers (19,20), spirometric values (21,22) and other techniques as forced oscillation, dynamic elastance (23) and unrestrained plethysmography (24) have been proposed as noninvasive methods to assess animal allograft function and rejection. However, the adoption of these techniques is limited by their complexity and previous studies have been conflicting in their conclusions (25).
The feasibility of LUS in rodents has been demonstrated by some authors (26) but the need for GA, intubation and shaving of the animals causes the loss of the potential non-invasive feature of this tool.
In this study, a new method to perform LUS examination in awake transplanted rodents is proposed for graft monitoring.
Methods
LUS was applied to native and graft lungs of rats after left orthotopic transplant.
The study was approved by the Research Ethics Committee of the University of Padua (n. 23/2014: 244; 13/10/2014). All procedures were conformed with the European legislation on animal research (27) and the NIH “Principles of Laboratory animal care” (28).
This study included a total of 13 rodents: 6 lung donors, 6 lung recipients and 1 control animal. All animals were 8- to 10-week-old Sprague-Dawley female rats (weight 250 to 350 g). Animals were single-housed in a pathogen-free environment, maintained on 12 h light/dark cycle, with unlimited access to food and water, constant temperature (22±2 °C) and humidity (50%±5%).
All experiments were carried out to minimize animal suffering and clinical signs were recorded 3 times a day using a dedicated evaluation system to recognize early on signs of distress (29,30).
Before surgery, animals were administered GA with intraperitoneal ketamine (100 mg/kg) and xylazine (4 mg/kg). Recipient rats received additional analgesia with buprenorphine (0.005 mg/kg) subcutaneously (sc) at induction and 3 times a day in the following 7 days. After surgery, rats were treated with gentamicin (10 mg/kg/day sc) and sub-optimal immunosuppressive treatment (cyclosporine A, 1.5 mg/kg sc) for 1 week. Animals were handled at least 3 times a day for 2 min by the dedicated experimenters to administer therapies.
The transplantation was performed using the cuff technique as described by Reis et al. (31). Briefly, the heart and lungs block were collected from the donor animal, the left lung was separated ex vivo and intravenous catheters were used to make cuffs for each pulmonary artery (PA), pulmonary veins (PVs) and bronchus. The chest cavity of the recipient animal was prepared with a left posterolateral thoracotomy through the fourth intercostal space, the hilum of the lung was dissected and the PA, PV and bronchus were identified. The left lung was removed from the chest cavity, a small incision was made on the ventral part of PA, PV and bronchus, hence each element was anastomosed by placing cuffs inside each of the corresponding structures. Once the lung was implanted, the native lung was excised. The ventilation and the perfusion of the graft were restored by removing clips from the left bronchus, PV and PA. The thoracotomy was closed and a pleural drainage tube connected to a syringe was introduced; the drainage tube was aspirated to return the pleural cavity to negative pressure and when the animal was breathing spontaneously the drainage tube was removed.
Rats were sacrificed ten weeks after lung transplant, if not earlier euthanized because of distress. After administration of GA, a median sternotomy was performed and the heart-lung block was collected for pathological examination.
LUS technique
The control animal was examined while awake with LUS twice, at 24-hour intervals, to record normal lung pattern clips and to exclude hypothetical ultrasound mediated tissue damage.
Transplanted rats were monitored with LUS on day 1, 5 and 10 after surgery, then once a week and finally two times on the day of sacrifice.
Multiple precautions were observed to minimize animal distress and permit the completion of the exam while awake. The examination room was illuminated with dim light; the examination table was covered with a surgical drape for the animal to grasp to; the ultrasound gel was heated to 37 °C using a bottle warmer and each animal was brought inside the room 15 minutes prior to testing for habituation to the environment. Then the rat was taken out of its transport cage, placed prone on the examination table and gently restrained by one examiner using his hands with the aid of a towel.
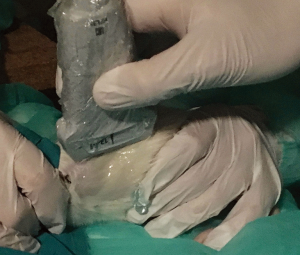
Ultrasound gel was liberally applied to its dorsal region with a massage motion to maximize air removal from the bristles. Hence, the thorax of the animal was examined using a linear transducer (EDGE, Fujifilm Sonosite, 13-6 MHz, HLF 38, USA) with the following settings: center frequency 6.8 MHz, depth 2.7 cm and mechanical index 1.3. Six-second clips were recorded. Using the vertebral column as initial reference point, the US beam was aligned to the sagittal plane. The procedure was performed both on the left (graft) and on the right (native) hemithorax. While examining the graft side, because of the relatively small size of the left lung, great care was taken to confirm the initial position of the probe and to avoid any erroneous scan of the bigger right native lung that partially fills the left thoracic cage (Figure 1).
At the end of the evaluation, all animals were dried of the remaining gel and returned to their cages. The time to complete the examination was recorded. The findings considered to be relevant for the monitoring of the transplant procedure were recorded and successively considered during the final pathological examination.
On the day of sacrifice, animals were first examined as described and again after administration of GA and shaving of the thorax.
The quality of LUS images was evaluated by two physicians considered expert in human LUS, who were blind to the source of the clips (examined subject and side). A four-level scoring system (1-poor; 2-limited; 3-good; 4-excellent quality) was applied, based on the presence of the following elements: 1-intelligible intercostal spaces; 2-lung sliding; 3-lung artifacts (A/B lines) and 4-diaphragmatic excursions (curtain sign). If the lung parenchyma appeared distinctly consolidated, the image was rated with a score of 4. To evaluate if there would be any improvement in the LUS technique as the study proceeded, times were compared between the first and second trimester of experiments.
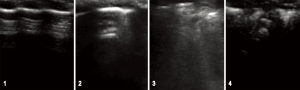
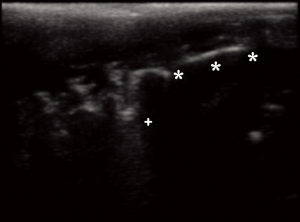
The LUS images were interpreted considering the following patterns: 1-normal pattern, with pleural sliding and A-lines; 2-isolated B-lines (no more than 2 B-lines per intercostal space) 3-multiple or coalescent B-lines (more than 2 B-lines per intercostal space or a light beam image) 4-consolidated parenchyma, without sliding and comparable to hepatic parenchyma density (32) (Figure 2).
Statistical methods
The quality of images was assessed twice by the two reviewers. Intra- and inter-rater agreement was graded using a weighted Cohen’s Kappa considering the distance between the paired values of the evaluation score. Median times in seconds to complete the examination were compared using the Wilcoxon-Mann-Whitney test. All tests were performed using Stata 13.0 (StataCorp, USA).
Results
The clips recorded from the control animal were referenced as normal lung pattern. For each animal both lungs were examined; the images were evaluated and interpreted considering them individually as well as comparing the graft to the native organ.
A total of 78 clips were recorded from transplanted rodents and their quality per se assessed by examiners blind to the source of the images (i.e., native or graft hemithorax).
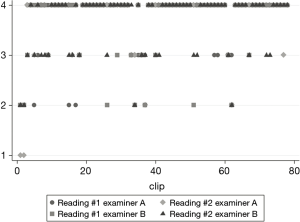
Median quality score of LUS was 3.66/4 for left hemithorax, 3.71/4 for native right side; the intra-rater weighted Cohen’s Kappa of each reviewer was 0.53 (P<0.01) and 0.65 (P<0.01). The inter-rater weighted kappa was 0.61 (P<0.01) (Figure 3).
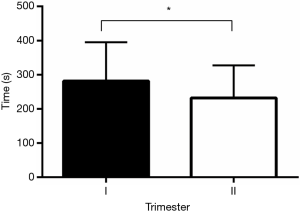
Median time to complete the examination was 233.0 seconds (IQR 142) for both lungs. By increasing the experience in performing LUS on rodents, the median time to get the clips of both lungs was lowered from 254.0 seconds (IQR 129.5) (first trimester of study) to 205.5 seconds (IQR 88.5) (second trimester of the study) (P<0.05) (Figure 4).
The overall score agreement on the day of sacrifice, between the clips obtained first with the animals awake and then under GA without bristles was 0.83 (Figure 5).
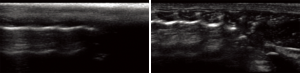
Over the course of the experiments, LUS was applied as a monitoring tool. Sliding of pleural line, B-lines and consolidations were noted (Figure 6). These patterns were identified considering the native lung of the same animal and the normal pattern acquired from the control animal.
For each animal, the ultrasound pattern on the day of sacrifice was compared to the macroscopic findings of the dissection and the results of the histological examination (Table 1, Figure 7).
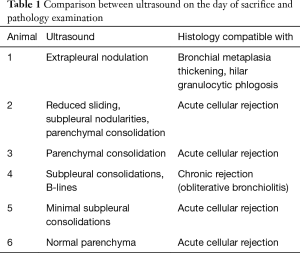
Full table
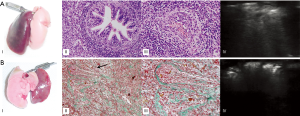
A broncho-pleural fistula (Figure 8), a diffuse bleeding of the graft, a fibrotic change of the graft with loss of function were diagnosed on LUS and then confirmed on autoptic examination.
During GA, a selective right bronchial intubation was detected on LUS considering the loss of sliding of the graft and promptly corrected. On the first day after transplantation, two rodents showed consolidations with a small number of B-lines in the graft that disappeared after the third day.
None of the animals showed any behavioral change after LUS examination suggestive of distress. The histological analysis of collected tissues did not show any lesion related to ultrasound.
Three animals had to be sacrificed before the stated end-point because of suffering; the reasons of this were: hemothorax with massive hemoptysis and pulmonary edema.
Discussion
In the last years, a great interest in LUS has been raised, especially relative to the pediatric population who could potentially get the most benefit from this non-invasive, radiation-free technique (33). In critical care patients, LUS has been proposed as a point-of care and bedside tool to detect several pathological conditions (32) with a confirmed clinical impact (15).
The interaction between ultrasounds, tissue and air generates artifacts that can be interpreted to get information about the changes in density of the lung parenchyma (14). B-lines arise from pleural lines and depend on the porosity of the tissue: when the lung parenchyma increases its density and reduces its porosity (i.e., more tissue and less air), the partial penetration of ultrasound generates vertical artifacts proportional in number to the density of the parenchyma (16).
Orthotopic left lung transplantation in the rat is an established experimental model to study organ dysfunction (34). Nevertheless, lack of a real time, noninvasive, repeatable, convenient technique to evaluate the graft function limits data collection. Micro-computer tomography, micro-magnetic resonance and micro-positron emission tomography have been described as validated diagnostic tools but the high costs of these technologies (from $300,000 to $2,000,000) limit their use (3). Moreover, the need for GA or deep sedation to immobilize the animals during the diagnostic procedure may affect tidal volume, induce atelectasis and limit diaphragmatic excursions: data could be misinterpreted and functional findings lost (35).
In this study, the use of LUS in awake rodents appeared to be a reliable and effective method to acquire information about lung parenchyma. Different LUS patterns were identified with good inter- and intra-rater agreement. It should be emphasized that it was possible to obtain dynamic images and related artifacts of the graft without the need for deep sedation, intubation and even bristles removal. Moreover, the graft could be compared to the contralateral native lung to obtain information about differences in sliding, parenchymal density and function.
The presence of edema, fibrosis, atelectasis, pleural effusion, hepatization or infiltrates could modify the LUS pattern; codification of these artifacts could give much anatomic and functional information about the graft.
In this study, it was possible to assess the increase of density of the graft but without identifying the specific etiology. The change of pattern, from A to B lines, could be explained as a primary graft dysfunction or as incomplete aeration of the graft just after the transplant procedure. The complete re-aeration of the graft was later monitored with the change of lung pattern and the disappearance of the B-lines. Because of the small sample size of this study and considering the complexity of the mechanism of graft dysfunction, it was not possible to establish the strength of the correlation between ultrasound and pathological findings. Nevertheless, the opportunity to obtain a simultaneous comparison between graft and native lung could offer important information about the graft function. A systemic infection that involves both lungs could be differentiated from an isolated graft dysfunction due to rejection or leakage of anastomosis; the improvement in graft aeration after therapy could be monitored daily with a rapid examination considering the disappearance of B-lines; graft failure that only minimally affects total lung function could be visualized in real time as a definite change to a consolidated pattern before histological examination. Moreover, LUS could provide information about the heart function: a bilateral increase of B-lines without lung consolidation could be interpreted as lung edema due to heart failure, mismatch between heart contractility and afterload or valvular dysfunction.
The time needed to complete the examination decreased over the course of the study, which could be explained by the increased experience gained by the examiners and the habituation of the animals to the procedure.
Several macroscopic findings that could be difficult to diagnose clinically in living animals were identified with LUS and successively confirmed with autoptic examination: a broncho-pleural fistula was recognized as a new-onset nodule between lung tissue and pleura (Figure 6); the diffuse bleeding of the graft showed as a fully consolidated nonfunctional lung; the fibrotic substitution of a graft appeared with a loss of sliding with reduction in size and improved echogenicity.
Moreover, the use of LUS on rodents was useful to guide the proper execution of the experimental procedures. One animal under GA on the day of sacrifice showed a unilateral loss of sliding; the position of the endotracheal tube was changed to few millimeters out until the sliding re-appeared. Indeed, the correct position of the endotracheal tube in small animals is always a tricky issue. The risk of selective intubation is high and the consequences could be dangerous for the animal (36). LUS technique could be helpful in reducing this risk and be applied to different species.
Ultrasound mediated lung damage has been described (37). In our study the LUS pattern has never changed during the exam as suggesting an ultrasound-related injury. Moreover, the histological examination of collected tissue did not show any microscopic alterations associated with ultrasound exposure as described by Miller et al. (38). The short time of exposure of the parenchyma to ultrasound, the low mechanical index, the bristles and the large amount of gel could have contributed in preserving the lungs from any possible ultrasound-induced damage. In contrast to the study of heart function in small animals, LUS technique is based on the study of artifacts and does not require a high frequency probe which could be associated with a higher risk of tissue damage (37).
The main limitation of this study was the small number of examined animals; to confirm the feasibility of this technique, it should be applied on a larger cohort to exclude anatomic and behavioral variability that could limit its adoption. Moreover, to study the rejection process, the inclusion of a similar number of syngeneic lung transplants could be considered. This would allow us to better understand the change in LUS pattern and evaluate how LUS can be applied to predict the graft dysfunction.
Conclusions
In this proof-of-concept study, LUS in awake rodents without shaving has been shown to be both feasible and safe. The images collected were of good quality and comparable to those obtained in anesthetized rats without bristles. The interaction between ultrasound and lung tissues generates artifacts that could be interpreted as in humans. The comparison between graft and native lung in the same animal appeared to be useful in assessing graft function and repeatable without risk of damage to the parenchyma. Animals did not show any signs of distress following the LUS examination. The time required for each procedure decreased with the number of examinations. This study suggests that LUS could be a promising technique to monitor in-vivo lung function in orthotopic lung transplant rodent model.
Acknowledgements
Funding: This work was supported by Fondazione Cariplo Ref. 2013-0943.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Research Ethics Committee of the University of Padua (n. 23/2014: 244; 13/10/2014).
References
- Marck KW, Wildevuur CR. Lung transplantation in the rat: I. Technique and survival. Ann Thorac Surg 1982;34:74-80. [Crossref] [PubMed]
- Marck KW, Piers DA, Wildevuur CR. Lung transplantation in the rat: II. lung perfusion scintigraphy in normal and left lung-transplanted rats. Ann Thorac Surg 1982;34:81-8. [Crossref] [PubMed]
- Flechsig P, Kratochwil C, Warth A, et al. A Comparison of microCT and microPET for Evaluating Lymph Node Metastasis in a Rat Model. Mol Imaging Biol 2016;18:243-8. [Crossref] [PubMed]
- Obert M, Ahlemeyer B, Baumgart-Vogt E, et al. Flat-panel volumetric computed tomography: a new method for visualizing fine bone detail in living mice. J Comput Assist Tomogr 2005;29:560-5. [Crossref] [PubMed]
- Kiley S, Cassara C, Fahy BG. Lung ultrasound in the intensive care unit. J Cardiothorac Vasc Anesth 2015;29:196-203. [Crossref] [PubMed]
- Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia*. Crit Care Med 2010;38:84-92. [Crossref] [PubMed]
- Volpicelli G, Skurzak S, Boero E, et al. Lung Ultrasound Predicts Well Extravascular Lung Water but Is of Limited Usefulness in the Prediction of Wedge Pressure. Anesthesiology 2014;121:320-7. [Crossref] [PubMed]
- Williamson JP, Grainge C, Parameswaran A, et al. Thoracic Ultrasound: What Non-radiologists Need to Know. Curr Pulmonol Rep 2017;6:39-47. [Crossref] [PubMed]
- Irwin Z, Cook JO. Advances in Point-of-Care Thoracic Ultrasound. Emerg Med Clin North Am 2016;34:151-7. [Crossref] [PubMed]
- Vizioli L, Ciccarese F, Forti P, et al. Integrated Use of Lung Ultrasound and Chest X-Ray in the Detection of Interstitial Lung Disease. Respiration 2017;93:15-22. [Crossref] [PubMed]
- Bouhemad B, Brisson H, Le-Guen M, et al. Bedside Ultrasound Assessment of Positive End-Expiratory Pressure-induced Lung Recruitment. Am J Respir Crit Care Med 2011;183:341-7. [Crossref] [PubMed]
- Goffi A, Pivetta E, Lupia E, et al. Has lung ultrasound an impact on the management of patients with acute dyspnea in the emergency department? Crit Care 2013;17:R180. [Crossref] [PubMed]
- Mayo P, Volpicelli G, Lerolle N, et al. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med 2016;42:1107-17. [Crossref] [PubMed]
- Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med 2009;28:163-74. [Crossref] [PubMed]
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014;20:315-22. [Crossref] [PubMed]
- Soldati G, Inchingolo R, Smargiassi A, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol 2012;38:1169-79. [Crossref] [PubMed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014;88:458-68. [Crossref] [PubMed]
- Irvin CG, Bates JHT. Measuring the lung function in the mouse: the challenge of size. Respir Res 2003;4:4. [Crossref] [PubMed]
- Yamane M, Sano Y, Nagahiro I, et al. Humoral immune responses during acute rejection in rat lung transplantation. Transpl Immunol 2003;11:31-7. [Crossref] [PubMed]
- Murata K, Iwata T, Nakashima S, et al. C4d Deposition and Cellular Infiltrates as Markers of Acute Rejection in Rat Models of Orthotopic Lung Transplantation. Transplantation 2008;86:123-9. [Crossref] [PubMed]
- Otulana BA, Higenbottam T, Ferrari L, et al. The use of home spirometry in detecting acute lung rejection and infection following heart-lung transplantation. Chest 1990;97:353-7. [Crossref] [PubMed]
- Van Muylem A, Mélot C, Antoine M, et al. Role of pulmonary function in the detection of allograft dysfunction after heart-lung transplantation. Thorax 1997;52:643-7. [Crossref] [PubMed]
- Takahashi A, Hamakawa H, Sakai H, et al. Noninvasive assessment for acute allograft rejection in a rat lung transplantation model. Physiol Rep 2014;2. [Crossref] [PubMed]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 2003;94:1297-306. [Crossref] [PubMed]
- Lundblad LK, Irvin CG, Adler A, et al. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol 2002;93:1198-207. [Crossref] [PubMed]
- Ma H, Huang D, Zhang M, et al. Lung ultrasound is a reliable method for evaluating extravascular lung water volume in rodents. BMC Anesthesiol 2015;15:162. [Crossref] [PubMed]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union 2010;L 276:33-79.
- NIH. Guide for the Care and Use of Laboratory Animals. Washington: National Academies Press, 2011.
- Morton DB. Refinement of in vivo tests. Dev Biol Stand 1999;101:187-93. [PubMed]
- Schuppli CA, Fraser D, McDonald M. Expanding the three Rs to meet new challenges in humane animal experimentation. Altern Lab Anim 2004;32:525-32. [PubMed]
- Reis A, Giaid A, Serrick C, et al. Improved outcome of rat lung transplantation with modification of the nonsuture external cuff technique. J Heart Lung Transplant 1995;14:274-9. [PubMed]
- Bouhemad B, Mongodi S, Via G, et al. Ultrasound for “Lung Monitoring” of Ventilated Patients. Anesthesiology 2015;122:437-47. [Crossref] [PubMed]
- Raimondi F, Rodriguez Fanjul J, Aversa S, et al. Lung Ultrasound for Diagnosing Pneumothorax in the Critically Ill Neonate. J Pediatr 2016;175:74-8.e1. [Crossref] [PubMed]
- Habertheuer A, Kocher A, Laufer G, et al. Innovative, simplified orthotopic lung transplantation in rats. J Surg Res 2013;185:419-25. [Crossref] [PubMed]
- Hedenstierna G, Lundquist H, Lundh B, et al. Pulmonary densities during anaesthesia. An experimental study on lung morphology and gas exchange. Eur Respir J 1989;2:528-35. [PubMed]
- Thomas JL, Dumouchel J, Li J, et al. Endotracheal intubation in mice via direct laryngoscopy using an otoscope. J Vis Exp 2014. [Crossref] [PubMed]
- Miller DL. Induction of Pulmonary Hemorrhage in Rats During Diagnostic Ultrasound. Ultrasound Med Biol 2012;38:1476-82. [Crossref] [PubMed]
- Miller DL, Suresh MV, Dou C, et al. Characterization of ultrasound-induced pulmonary capillary hemorrhage in rats. Microvasc Res 2014;93:42-5. [Crossref] [PubMed]