TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer
Introduction
Conventional transbronchial needle aspiration (TBNA) has been used for over 30 years to obtain histologic and cytological specimens from lymph nodes in the chest, with sensitivities approaching 80-90% (1-8). Recently endobronchial ultrasound (EBUS) TBNA has demonstrated even higher sensitivities among experts (8,9). However EBUS-TBNA is more complicated, requires additional training, less well tolerated by patients and more costly than conventional TBNA (c-TBNA) (10). A prospective comparison study was carried out to determine the efficacy of TBNA with and without EBUS in the diagnosis and staging of mediastinal and hilar malignant adenopathy.
Methods
A total of 287 patients with mediastinal and/or hilar lymphadenopathy presenting for diagnosis and/or staging were included in the study. All participating bronchoscopists were trained extensively by the senior investigator, KPW, and demonstrated competency in conventional TBNA and EBUS-TBNA prior to commencing study. Equal numbers of punctures (1 to 3 passes per lymph node) were performed at the target lymph node stations using conventional TBNA techniques followed by EBUS-TBNA at the same sites and by the same bronchoscopist on the same patient. In all cases, TBNA was performed first without EBUS so that there would be no puncture sites to guide the bronchoscopist. When nodal staging was the goal, the lymph node station that would give the highest stage was sampled first, followed by stations representing descending stages (i.e., N3-N2-N1 lymph node biopsy order). Rapid on-site cytology for adequacy of specimens was not used. Specimens were then smeared for cytology on slide and sent for cytospin and cellblock for any additional immunohistochemical or molecular marker studies. The adequacy of the specimen was graded by the quantity of lymphocytes and the quality of diagnostic tissue. A pathologist who was not part of the study team reviewed the slides and wrote a formal interpretation of each nodal station. The diagnostic results of each procedure and each lymph node biopsy station with and without EBUS were recorded and compared. Only the biopsies that were diagnostic for malignancy are presented in this report. Statistical analysis was done using STATA software and Pearson’s chi squared test. The study was approved by the Institutional Review Boards of enrolling hospitals.
Results
From 27 NOV 2006 to 26 APR2013, 287 patients were studied. In 253 patients at least one pair of specimens were obtained by conventional TBNA and EBUS-TBNA. In 83 of these cases, malignancy was diagnosed. Of these 83 malignant cases, c-TBNA diagnosed 64 and EBUS-TBNA diagnosed 62. c-TBNA and EBUS-TBNA were both positive in 50 of these patients with exclusive positivity by c- TBNA in 14 patients and exclusive positivity by EBUS-TBNA in 12 patients (Table 1). Among the 83 patients with a diagnosis of a malignancy there was also no significant difference seen between the diagnostic yield of conventional TBNA and EBUS-TBNA (Table 2). There was a significant difference in the number of positive (diagnostic for malignancy) stations sampled by c-TBNA and EBUS-TBNA (Table 3).
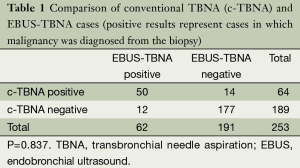
Full table

Full table

Full table
Although 287 patients were studied only 253 patients had at least one pair of specimens obtained by c-TBNA and EBUS-TBNA. In 34 patients EBUS was not completed due to patient lack of tolerance of the procedure. All of these procedures were performed under local anesthesia with intravenous moderate sedation. Often, the patient would tolerate the normal bronchoscopy with c-TBNA well, but when the larger diameter EBUS scope with the rigid transducer tip was used the patients became too uncomfortable to continue. Patient intolerance accounted for the large number of stations that were not sampled by EBUS-TBNA compared with c-TBNA.
Discussion
The recent development of EBUS to guide TBNA (EBUS-TBNA) has generated special attention and interest in the TBNA technique and has been shown to be more reliable and sensitive than conventional TBNA in some centers (11,12). EBUS-TBNA distinguishes itself from conventional TBNA in several significant ways. First is the ability to visualize and locate the target lymph node with ultrasound and then to perform the needle aspiration with real time ultrasound guidance. Second, the needle designed for the EBUS bronchoscope is longer and stiffer, making penetration through the tracheobronchial wall easier and allows for deeper penetration of the needle through the lymph node (13). Third, the needle apparatus is fixed to the scope at the working channel and the needle is moved independently, unlike conventional TBNA where the needle is moved with the sheath. By fixing the length of the sheath, the needle is prevented from being pushed too far out during the puncture attempt which is one of the most common problems when performing conventional TBNA. Another advantage for the EBUS-TBNA apparatus is that the needle sheath is locked to the scope and thus, the needle will not be pushed back into the scope channel when resistance is met. This is the second most common problem in conventional TBNA. Another major difference is the exit angle of the needle at the distal port of the working channel, where the EBUS bronchoscope automatically positions the needle at a good puncturing angle relative to the airway wall even with a straightened scope.
Despite these unique qualities of the EBUS scope and needle, the methodology for EBUS-TBNA is essentially the same as for conventional TBNA with only a few exceptions. First, location of the puncture site is identified visually using the endoscopic view of the airway and the natural landmarks that correlate to the CT image of the mediastinum as described in 1994 (14). This map included endobronchial, anatomic, and CT correlation for 11 of the most common locations for mediastinal adenopathy that can be reached from the airways. While this map was created for the bronchoscopist performing TBNA to enhance their ability to successfully locate and biopsy the target lymph nodes, it correlates very closely to the latest IASLC staging system (15). Unlike biopsy from the esophagus, the airways have distinctive landmarks to identify the areas where the lymph nodes consistently reside. These landmarks are very reliable and do not require ultrasound guidance to locate.
In 12 cases EBUS-TBNA was the only diagnostic specimen that was positive. It is reasonable to assume that the ultrasound guidance would be beneficial in lymph nodes located farther from endobronchial anatomic landmarks as in the case of a mid paratracheal lymph node (2R and 2L). Advantage in sampling smaller lymph nodes is also expected with EBUS real time visualization technology. Despite these 12 cases, it is interesting that in 14 different cases, conventional TBNA was the only diagnostic modality. No specific reason is obvious for this other than the patient tolerance of the procedure under moderate sedation or the larger and less flexible EBUS scope. In many of these cases only one puncture was made by each method, which should favor EBUS-TBNA punctures. It may also represent the learning curve of EBUS TBNA in which we are comparing a 30-year experience with conventional TBNA of the primary bronchoscopist versus a 3-year experience with EBUS-TBNA. With appropriate training and skill in both conventional TBNA and EBUS-TBNA, the difference between the two techniques may be minimized. In fact it is most interesting that in our first 100 patients, conventional TBNA was exclusively positive in 7 cases with zero cases exclusively positive by EBUS-TBNA. In the second set of 100 cases, there were 5 cases exclusively positive by conventional TBNA and 7 exclusively positive with EBUS-TBNA. This ratio shift between the two techniques as experience with EBUS-TBNA increased may support this speculation (16).
While there was no significant difference in the diagnosis of malignant cases, there were a significantly greater diagnostic percentage of overall positive (malignant) stations sampled by EBUS-TBNA. This is not surprising, as real-time visualization of the lymph node with EBUS should allow the bronchoscopist to puncture the target in fewer attempts than with c-TBNA, which is based off of anatomic landmarks and static CT scan correlation.
Using the EBUS bronchoscope to visualize the anatomy behind the tracheobronchial wall and the needle within the target lymph node is reassuring and often gives more detailed information about the lymph node that was not evident on CT scan. Despite this feature, the most important information needed for diagnosis and staging with any TBNA technique is still the cytology and histology. Any bronchoscopist with enough experience with EBUS-TBNA and rapid on site cytology (ROSE) knows very well that seeing the needle in the lymph node does not guarantee a diagnostic specimen. EBUS imaging has demonstrated and confirmed the consistency of endobronchial and CT correlation for locating lymph nodes and has also demonstrated clearly the limitations that we have yet to overcome in TBNA. Understanding the variables in TBNA that prevent us from achieving closer to 100% yield on every pass that we visualize the needle in the lymph node is required for future improvements. Some combination of the instrument (scope and needles), the technique, or target lesion consistency or characteristics must play a role.
Conclusions
The diagnostic yield of c-TBNA and EBUS-TBNA performed sequentially under moderate sedation in cases of malignancy were not significantly different in our study. Recommendations for current practice depend on individual centers and bronchoscopist comfort level with TBNA (with or without EBUS). For the majority of lung cancer cases where diagnosis and staging is required with mediastinal adenopathy seen on CT, it is likely that conventional TBNA will be able to achieve the diagnosis and stage if EBUS is not available or if the patient will not tolerate the EBUS procedure. For those who desire maximal assurance that successful biopsy was achieved at the procedure, the addition of ROSE offers the most assurance but even that is not a guarantee since half of the slides are processed later and cellblock may contain specimens not present on specimens from the initial slides. The costs of all of these additional reassurances are significant and many institutions may not be able to afford the EBUS systems or ROSE. For centers that are comfortable with conventional TBNA, performing conventional first and only escalating to EBUS when conventional is non-diagnostic is supported by our findings.
As a bronchoscopist becomes more comfortable with the anatomy and TBNA technique, advancement from exclusive EBUS-TBNA to supplementing their practice with conventional TBNA will offer the greatest spectrum of care to the patients and allow for a more comfortable procedure with less sedation, lower costs, and similar yields if patient selection is matched with the bronchoscopist comfort level and skill in TBNA.
The authors thank Ms. Chae Striffolino and Ms. Tamara Moore from Nursing for their assistance in the study.
The authors have no disclosures or conflicts of interest. The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis 1983;127:344-7. [PubMed]
- Wang KP, Brower R, Haponik EF, et al. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest 1983;84:571-6. [PubMed]
- Wang KP. Flexible transbronchial needle aspiration biopsy for histologic specimens. Chest 1985;88:860-3. [PubMed]
- Welker JA, Alattar M, Gautam S. Repeat needle biopsies combined with clinical observation are safe and accurate in the management of a solitary pulmonary nodule. Cancer 2005;103:599-607. [PubMed]
- Patel NM, Pohlman A, Husain A, et al. Conventional transbronchial needle aspiration decreases the rate of surgical sampling of intrathoracic lymphadenopathy. Chest 2007;131:773-8. [PubMed]
- Wang KP, Gonullu R, Baker R. Transbronchial Needle Aspiration Versus Transthoracic Needle Aspiration in the Diagnosis of Pulmonary Lesions. Journal of Bronchology 1994;1:199-204.
- Low A, Medford AR. Endobronchial ultrasound-guided transbronchial needle aspiration. Rev Recent Clin Trials 2013;8:61-71. [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [PubMed]
- Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604-7. [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [PubMed]
- Medford AR. Convex probe endobronchial ultrasound: pitfalls, training and service issues. Br J Hosp Med (Lond) 2011;72:312-7. [PubMed]
- Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest 1994;106:588-93. [PubMed]
- Wang KP, Browning R. Transbronchial needle aspiration with or without endobronchial ultrasound. Thoracic Cancer 2010;1:87-93.
- Medford AR. Learning curve for endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2012;141:1643-author reply 1643-4. [PubMed]


