Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis
Introduction
Lung cancer is one of the most common malignant cancers both in men and women, and the leading cause of cancer death worldwide (1). It is reported 222,500 people received a new diagnosis of lung cancer and 155,870 patients died of lung cancer in United States, 2017 (1). Lung cancer can be divided into two types: small cell lung cancer (SCLC) and non-small lung cancer (NSCLC). Despite great progresses made in early diagnosis and targeted therapy, the prognosis of lung cancer still remains poor, with a 5-year survival less than 17% (2). Lacking prognostic parameters are one important reason for the disappointing prognosis (3). Therefore, it is critical for us to find efficient biomarkers for prognosis prediction in order to improve clinical outcomes.
Prognostic nutritional index (PNI), calculated on the basis of serum albumin level and total lymphocyte count in peripheral blood, is a widely used nutritional and immunological index. It was initially proposed to stratify operative risk and evaluate perioperative nutritional and immunological conditions (4,5). Increasing evidences showed that the preoperative nutritional and immunological status was not only affect short-term postoperative complications but also closely related to long-term outcome of cancer patients (6,7). To date, numerous studies have reported preoperative or pretreatment PNI status predicts prognosis in various cancer types, including colorectal cancer (8,9), esophageal cancer (10), gastric cancer (11), bladder cancer (12), ovarian cancer (13) and so on (14-18). Previous researches also explored the relationship between PNI and lung cancer prognosis. However, those results failed to draw a convincing conclusion owing to relatively small sample size in each single study.
To clarify the prognostic and clinical impact of PNI in lung cancer, we conducted a meta-analysis of the current published studies on PNI and survival of lung cancer.
Methods
Search strategy
We conducted a systematic and comprehensive search in PubMed, EMBASE, and Web of Science, without restriction to language and race. Searching terms included “prognostic nutritional index”, “PNI”, “lung cancer”, “lung carcinoma”, “lung neoplasms”, “non-small cell lung cancer”, “NSCLC”, “small cell lung cancer” and “SCLC”. Besides, we manually checked reference lists of relevant studies to obtain potential eligible articles. The search formula of each search web site was given as Supplemental data.
Inclusion and exclusion criteria
We selected studies based on the following criteria: (I) the diagnosis of lung cancer was pathologically confirmed; (II) PNI was measured prior to treatment; (III) overall survival (OS) of PNI in lung cancer was available. Studies unrelated to lung cancer, published as reviews or conference abstracts, and without enough data for HRs and CIs were excluded. Only the most comprehensive study was enrolled if articles report data from the same population.
Data extraction
Two authors (DL and XY) reviewed eligible studies and extracted data independently. Disagreement was resolved by consulting to the third author (JL). Information extracted from the studies was the following: first author of the study, publication year, country, sample size; clinical features covering gender, age, histological type, tumor stage, cut-off value of PNI, medium follow up time; HR and CI for OS using multivariate analysis in each study. PNI was calculated using the formula: 10 × serum albumin value (g/dL) + 0.005 × total lymphocyte count (per mm3) in the peripheral blood (5). Items that could not be obtained were described as “not available (NA)”.
Quality assessment
We adopted the Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluate the quality of studies (19). Two authors (DL and XY) accomplished the work and the third author settled all differences (JL). NOS adopted star system with scoring 0–9. Each study was judged on three perspectives: study selection, comparability assessment, ascertainment of exposure and outcome. Studies scored not less than 6 were considered to have a good quality.
Statistical methods
We used pooled hazard ratio (HR) and its corresponding 95% confidence interval as effect measures to assess the impact of PNI on OS. To evaluate the relationship between PNI and clinical characteristics, odds ratios (ORs) and their 95% CI was applied. As all enrolled studies reported HR and 95% CI of OS directly both in univariate and multivariate analysis, we chose the latter since clinical outcomes in lung cancer were influenced by confounding factors. Q-test and I2 index were used to assess the heterogeneity across studies. When the I2≤50% or/and P<0.10, we used fixed effect model, otherwise, a heterogeneity was indicated and a random effect model was adopted (20). To evaluate the stability of the results, we conducted sensitivity analysis by omitting study sequentially. The Begg’s test was used to assess the publication bias (21,22). All P values were 2-sided; P<0.05 was considered statistically significant. All statistical analyses were performed using Stata 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Study selection process
After a comprehensive search from electronic databases, we identified a total of 224 articles as potential eligible studies. One hundred and ninety articles remained after duplication removed. Subsequently, titles and abstracts were carefully reviewed and 169 articles were excluded because of unrelated to the present study. We further assessed full-texts of 19 articles, 9 articles were ruled out due to conference abstract (n=4), insufficient data on survival (n=4) and unavailable PNI data (n=1). Finally, we enrolled 10 articles in the present meta-analysis (23-32) (Figure 1).
Study characteristics
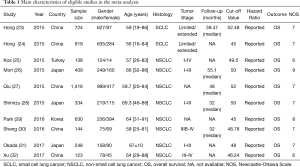
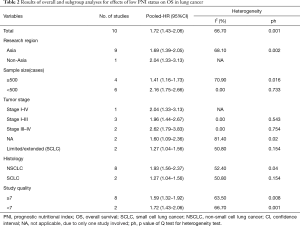
In the meta-analysis, data from 10 studies including 5,085 lung cancer patients was included. All those studies were published during 2015 to 2017. Only one study was from Turkey, and the rest nine studies were from Asian population. The smallest study population was 144 while the largest one was 1,416. The age of the patients ranged from 16 to 88, and the overall proportion of males was 66.86%. Furthermore, two studies explored PNI prognostic value among SCLC patients while the other eight studies did among NSCLC patients. For studies among NSCLC population, one enrolled patients with all stages, three based on patients with stage I–III and two studies focused on advanced lung cancer (stage III–IV), with the remaining two studies failed to provide stage information of patients. The cut-off value of PNI varied from 46.24 to 52.48. Moreover, all studies included directly reported HR and 95% CI of OS in multivariate analysis (Table 1).
Full table
Association between PNI and OS
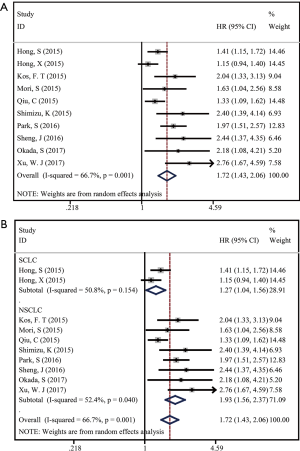
All studies investigated the relationship between PNI status and OS of lung cancer. We adopted a random effect model to calculate the pooled HR due to significant heterogeneity (I2=66.7%, P=0.001). The result revealed low PNI was significantly related to unfavorable OS in lung cancer (HR =1.72; 95% CI, 1.43–2.06; P=0.000; Figure 2A). Subgroup analysis stratified by histology (SCLC or NSCLC) was performed. The pooled HR in NSCLC patients (HR =1.93; 95% CI, 1.56–2.37; P=0.000) was higher than that in SCLC patients (HR =1.27; 95% CI, 1.04–1.56; P=0.021) (Figure 2B), which demonstrated that the prognostic impact of PNI in NSCLC patients was stronger than that in SCLC patients. Further, subgroup analysis by TNM stage indicated that low PNI status predicted shorter OS in early NSCLC patients (stage I–III, HR =1.96; 95% CI, 1.44–2.67; P=0.01). When it came to advanced NSCLC patients, only two studies involved in the analysis with a pooled HR of 2.62 (95% CI, 1.79–3.83; P=0.000).
Other subgroup analyses stratified by research region (Asia vs. Non-Asia), sample size (≥500 vs. <500) and study quality (NOS score ≥7 vs. <7) were later fulfilled. These pooled results confirmed the prognostic value of low PNI on OS in lung cancer (Table 2).
Full table
Association between PNI and clinical characteristics
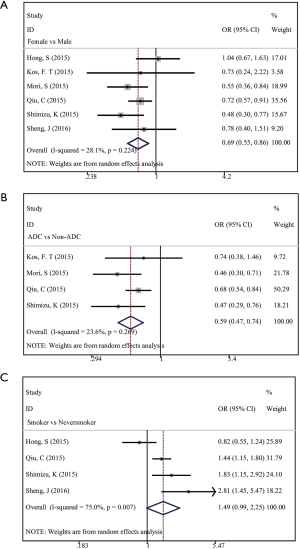
We analyzed the correlation between low PNI status and clinical characteristics including gender (female vs. male), smoking status (smoker vs. never smoker), and histology (adenocarcinoma versus non-adenocarcinoma). There were 3,155 cases from 6 studies, 2,598 cases from 4 studies and 1646 cases from 4 studies involved, respectively. We identified that PNI was significantly associated with gender (HR =0.69; 95% CI, 0.55–0.86; P=0.001; Figure 3A) and histology (HR =0.59; 95% CI, 0.47–0.74, P=0.000; Figure 3B). However, no significant association was found between PNI and smoking status (HR =1.49; 95% CI, 0.99–2.25; P=0.056; Figure 3C). Our results indicated that the incidence of low PNI was significantly lower in female patients than in male ones. Similarly, a significantly lower incidence of low PNI was observed in patients with adenocarcinoma compared with those with non-adenocarcinoma.
Sensitivity analysis
We performed Sensitivity analysis by omitting one study at a time and calculate the combined HR. The result showed the pooled HR was not significantly affected by the exclusion of any single study (Figure 4), which demonstrated that the conclusion of our meta-analysis on OS is reliable.

Publication bias
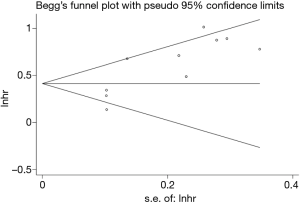
To evaluate publication bias, we carried out Begg’s funnel plot. No significant publication bias was found (P=0.210; Figure 5).
Discussion
The present meta-analysis aimed to assess the association between PNI, clinical characteristics and OS of lung cancer. We found that PNI was an independent indicator for OS in lung cancer. In addition, PNI was significantly related to clinical characteristics including gender and histology. To the best of our knowledge, this is the first meta-analysis that comprehensively discussed prognostic and clinical value of PNI in lung cancer.
We found that low PNI was an indicator for shorter OS in lung cancer, especially among NSCLC patients. Recent studies also reported that low PNI was an unfavorable marker for prognosis in several solid tumors. Nakatani and his colleagues found esophageal cancer patients undergoing neoadjuvant chemotherapy in low preoperative PNI status had a higher risk of recurrence and poorer survival (10). Kang and coworkers reported in renal cell carcinoma that monitoring of dynamics change of pre/postoperative PNI helped predict postoperative complications and long-term survival rate (7). Moreover, two recent meta-analyses explored the prognostic value of PNI in gastric and colorectal cancer, respectively. It turned out both of the two studies concluded low PNI suggested poor OS in the above two tumor types (33,34). Those evidences were consistent with our finding. Additionally, in the current meta-analysis, HRs we extracted to calculate the pooled HR in order to assess the impact of PNI on OS were all directly reported from multivariate analysis in each research, which possessed adjustment for confounding risk factors and thus added reliability to the results regardless of the existence of inter-study heterogeneity. On the other hand, we accomplished subgroup analysis stratified by histology, TNM stage, research region, sample size and study quality, which further confirmed the prognostic value of PNI in lung cancer and helped us recognize specific subset of patients who would gain greater OS benefit from PNI in lung cancer population. The result of the subgroup analysis by histology indicated that low PNI harbored greater predictive power of OS in NSCLC group than in SCLC group. Given that there were only 2 studies concerning impacts of PNI on OS in SCLC population, more studies will be needed to further elucidate the issue and to throw lights on the mechanisms behind. In addition, subgroup analysis based on tumor stage showed PNI was associated with OS both in early stage lung cancer and in advanced lung cancer. Early stage lung cancer patients in low PNI status had a 96% relative increase in hazard of death compared with those in high PNI status, while in advanced lung cancer patients in low PNI status that relative increase in hazard of death was 162%. The above results may partly explained by the fact that early-stage patients would more likely to receive surgical therapy, and in this population PNI would have less predictive effect compared to advanced-stage who usually receive chemotherapy. Further well-designed and prospective studies were needed to confirming our findings.
Mechanisms underlying the association between PNI and OS can be the following. First of all, PNI is calculated based on serum albumin and lymphocyte count. Lymphocytes play a fundamental role in cell-mediated immunity in various cancers and can reflect systemic inflammation condition of cancer patients (35). As previous data suggested, inflammations was closely associated with carcinogenesis and tumor progression by promoting the proliferation, migration, immune escape and chemoresistance of tumor cells (36-38). Furthermore, level of albumin in PNI shows the nutritional status of cancer patients. Low albumin level is related to malnutrition and weight loss (39), which can result in a poor OS and increased cancer-related mortality (40,41). In a review examined association between albumin and OS for various cancer types, nine of the total 10 involved lung cancer studies found high preoperative albumin level indicated improved survival (42). Taken together, on the basis of two simple, objective and inexpensive laboratory indices, PNI harbored promising prognostic value for lung cancer.
When exploring the relationship between PNI and clinical characteristics, PNI was found related to gender and histology. The result indicated that low PNI status was less frequent in female lung cancer patients than in male ones. Similarly, low PNI status was less likely to exist in patients with adenocarcinoma than in those with non-adenocarcinoma. Considering the limited amount of studies included, these results needed to be confirmed by future researches.
Several limitations must be noticed in this meta-analysis. First, significant heterogeneity was found when investigating effect of PNI in OS, which can be caused by different cancer types, sample size, cut off value and so on. However, association between PNI and OS obtain the same results after subgroup analysis were carried out, and remove of any single study did not significantly affect the pooled HR in sensitivity analysis, all indicating that the results were quite reliable. Second, the majority of included studies were from Asian countries, suggesting the result was more suitable for Asian patients, whether it can be applied to other population remains unknown. Third, considering that studies with negative results may tend to have less chance to be published, potential selection bias can still exist. Thus, large-scale, multicenter and well-designed studies are required to verify and expand on our conclusion.
Conclusions
Low PNI was an indicator for shorter OS in lung cancer, especially among NSCLC patients. The current meta-analysis indicated that PNI can help further stratify risk of death and predict prognosis of lung cancer in clinical practice, thus, can be used as a supplementary tool to the present prognosis predicting systems including TNM staging system. Further large-scale, multicenter and well-designed studies are required to verify and expand on our conclusion.
Acknowledgements
Funding: This work was supported by the Transformation Projects of Sci-Tech Achievements of Sichuan Province (2016CZYD0001), and the Sci-Tech Support Program of Science and Technology Department of Sichuan Province (2016SZ0073).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-e29S.
- Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160-7. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis Colon Rectum 2015;58:1048-57. [Crossref] [PubMed]
- Kang M, Chang CT, Sung HH, et al. Prognostic Significance of Pre- to Postoperative Dynamics of the Prognostic Nutritional Index for Patients with Renal Cell Carcinoma Who Underwent Radical Nephrectomy. Ann Surg Oncol 2017;24:4067-75. [Crossref] [PubMed]
- Schwegler I, von Holzen A, Gutzwiller JP, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 2010;97:92-7. [Crossref] [PubMed]
- Nazha B, Moussaly E, Zaarour M, et al. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J Gastrointest Surg 2015;7:370-7. [Crossref] [PubMed]
- Nakatani M, Migita K, Matsumoto S, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Saito H, Kono Y, Murakami Y, et al. Influence of prognostic nutritional index and tumor markers on survival in gastric cancer surgery patients. Langenbecks Arch Surg 2017;402:501-7. [Crossref] [PubMed]
- Peng D, Gong YQ, Hao H, et al. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival with Bladder Cancer after Radical Cystectomy: a retrospective study. BMC Cancer 2017;17:391. [Crossref] [PubMed]
- Zhang W, Ye B, Liang W, et al. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep 2017;7:9548. [Crossref] [PubMed]
- Miao J, Xiao W, Wang L, et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol 2017;143:1263-73. [Crossref] [PubMed]
- Lee SH, Chung MJ, Kim B, et al. The Significance of the Prognostic Nutritional Index for All Stages of Pancreatic Cancer. Nutr Cancer 2017;69:512-9. [Crossref] [PubMed]
- Fan L, Wang X, Chi C, et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017;77:1233-41. [Crossref] [PubMed]
- Haraga J, Nakamura K, Omichi C, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol 2016;5:567-74. [Crossref] [PubMed]
- Du XJ, Tang LL, Mao YP, et al. Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. J Transl Med 2015;13:364. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629-34. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol 2015;36:3389-97. [Crossref] [PubMed]
- Hong X, Cui B, Wang M, et al. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med 2015;236:297-304. [Crossref] [PubMed]
- Kos FT, Hocazade C, Kos M, et al. Assessment of Prognostic Value of "Neutrophil to Lymphocyte Ratio" and "Prognostic Nutritional Index" as a Sytemic Inflammatory Marker in Non-small Cell Lung Cancer. Asian Pac J Cancer Prev 2015;16:3997-4002. [Crossref] [PubMed]
- Mori S, Usami N, Fukumoto K, et al. The Significance of the Prognostic Nutritional Index in Patients with Completely Resected Non-Small Cell Lung Cancer. PLoS One 2015;10. [Crossref] [PubMed]
- Qiu C, Qu X, Shen H, et al. Evaluation of Prognostic Nutritional Index in Patients Undergoing Radical Surgery with Nonsmall Cell Lung Cancer. Nutr Cancer 2015;67:741-7. [Crossref] [PubMed]
- Shimizu K, Okita R, Saisho S, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol 2015;13:291. [Crossref] [PubMed]
- Park S, Lee SH, Suh B, et al. Nutritional status in the era of target therapy: Poor nutrition is a prognostic factor in non-small cell lung cancer with activating epidermal growth factor receptor mutations. Korean Journal of Internal Medicine 2016;31:1140-9. [Crossref] [PubMed]
- Sheng J, Yang YP, Ma YX, et al. Low Prognostic Nutritional Index Correlates with Worse Survival in Patients with Advanced NSCLC following EGFR-TKIs. PLoS One 2016;11. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Clinical Significance of Prognostic Nutritional Index After Surgical Treatment in Lung Cancer. Ann Thorac Surg 2017;104:296-302. [Crossref] [PubMed]
- Xu WJ, Kang YM, Zhou L, et al. Zhonghua Zhong Liu Za Zhi 2017;39:146-9. [Clinical application value of prognostic nutritional index for predicting survival in patients with advanced non-small cell lung cancer]. [PubMed]
- Yang Y, Gao P, Chen X, et al. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget 2016;7:58543-52. [PubMed]
- Yang Y, Gao P, Song Y, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol 2016;42:1176-82. [Crossref] [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. [Crossref] [PubMed]
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759-71. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 2014;110:1409-12. [Crossref] [PubMed]
- Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol 2011;23:322-30. [Crossref] [PubMed]
- Fruchtenicht AV, Poziomyck AK, Kabke GB, et al. Nutritional risk assessment in critically ill cancer patients: systematic review. Rev Bras Ter Intensiva 2015;27:274-83. [PubMed]
- Deme D, Telekes A. Prognostic importance of albumin in oncology. Orv Hetil 2018;159:96-106. [Crossref] [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]