
Patient and procedural features predicting early and mid-term outcome after radical surgery for non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide and surgical resection remains the standard treatment for the early disease stages (1). Tobacco smoking is not only the key driver of lung cancer but also of chronic obstructive pulmonary disease (COPD) as well as cardiovascular and cerebrovascular diseases (2). Therefore, patients scheduled for lung cancer resection often present a combination of these chronic diseases whose presence and severity increase the risk of major postoperative complications (3). Procedural factors such as excessive fluids infusion and overstretching bronchoalveolar tissues with elevated inspiratory pressure and/or tidal volume may generate edema and inflammation that contribute to postoperative cardiovascular and pulmonary complications (PCVCs and PPCs) (4).
Several indices of aerobic fitness have been shown useful to predict early complications but also later survival and quality of life after major surgery (5). By providing breath-by-breath measurements of oxygen uptake (VO2), carbon dioxide output (VCO2) and ventilation (VE), cardiopulmonary exercise testing (CPET) has emerged the gold standard method for assessing the severity of cardiovascular and pulmonary diseases and for selecting suitable candidates for lung cancer resection (6). A peakVO2 less than 15–16 mL/kg/min has been shown predictive of a higher occurrence of cardiovascular and pulmonary complications (7,8). Importantly, reliance on peakVO2 as a predictor of major complications has recently been questioned in patients with COPD undergoing lung cancer resection (9). Another CPET-derived parameter, the slope (or ratio) of ventilation to carbon dioxide [VE/VCO2]), has been shown effective in predicting early mortality, cardio-pulmonary complications and survival at one-year after lung cancer surgery (10). Likewise, high VE/VCO2 slope is a well-known predictor of survival in patients with heart failure, pulmonary hypertension and idiopathic pulmonary fibrosis (11).
Consistent with the principles of fast-track surgery, risk-minimizing strategies including preoperative patient optimization as well as intraoperative goal-directed hemodynamic control, protective lung ventilation and smaller thoracic incisions or video-assisted thoracic surgery (VATS), have been implemented in the management of thoracic surgical patients (12). Given these recent improvements, there is a need to reevaluate all potential risk factors that may influence postoperative clinical outcome. Therefore, we performed a post-hoc analysis of data collected in the Lung Cancer Rehabilitation Study (LCRS) (13) and we questioned whether preoperative CPET parameters, in addition to preoperative clinical and biological factors, as well as intraoperative features, were associated with early cardio-pulmonary complications and later survival.
Methods
Study design and patient selection
The LCRS was an open blinded randomized controlled trial, registered at the ClinicalTrials.gov website (NCT01258478), and conducted at the University Hospital of Geneva and the Hospital of Valais between October 2011 and October 2014. The study was approved by the institutional ethics committee of the University Hospital of Geneva (protocol number: 06–225).
All adult patients with non-small cell lung carcinoma (NSCLC), stage IIIA or less (eligible for surgical cure), documented by CT-scan or Positron Emission Tomography CT scan, were screened for eligibility. Exclusion criteria consisted in any contraindication to perform CPET (e.g., clinically limiting or untreated heart disease, severe pulmonary hypertension) or inability to adhere to a rehabilitation program (e.g., limiting comorbidities, psychiatric condition, osteoarthritis impeding cycling). Consenting eligible patients were allocated to usual care or a rehabilitation intervention (2–3 weekly sessions of high-intensity interval training) that was performed over 2–3 weeks preceding surgery.
Perioperative management, measurements and study endpoints
Preoperative pulmonary function tests included lung volumes and carbon monoxide diffusion capacity.
Lung resections were performed by muscle-sparing thoracotomy or VATS. Standardized perioperative interventions included antibiotic prophylaxis, restrictive fluid management, thoracic epidural analgesia or wound infiltration and protective lung ventilation (12). All patients were extubated in the operating room, admitted in the postanesthesia care unit and transferred 3 to 6 hours later into the surgical ward. Postoperative physiotherapy consisted in deep breathing, coughing exercise and early ambulation.
A six minute walk test (6MWT) was conducted by physiotherapists and was followed by a symptom-limited CPET on an upright electronically braked cycle ergometer (SensorMedics Model 2200 SP; Yorba Linda, CA). Peak heart rate, peak work rate and peakVO2 were determined as the highest averaged value over 30 s. Derived measurements included the ratio of VE/VCO2 calculated at the respiratory compensation point, the peak oxygen pulse and the anaerobic threshold.
Measurements and study endpoints
Preoperative clinical, functional and biological data, as well as intraoperative surgical, hemodynamic and ventilatory data, were extracted from the electronic medical files. In addition to standard blood tests, N terminal pro-brain natriuretic peptide (NT-pro-BNP) was measured preoperatively. The 6MWT and CPET were performed twice (at enrollment and 1–2 days before surgery), the last results were included for analysis and a positive response to rehabilitation was defined by meaningful changes in VO2peak (≥10%) or in 6MWT (≥30 m) between the first and the second preoperative measurements.
Study endpoints were the early mortality rate (in-hospital and/or 30-day), the incidence of major PCVCs and PPCs and survival after surgery. The modified version of the Thoracic Mortality and Morbidity (TMM) classification system was used to report any serious adverse events that occurred during the postoperative hospital stay (14). Survival was reported by directly contacting the patient or his physician (up to 4 years after surgery).
Statistical analysis
Perioperative clinical, functional and surgical characteristics of patients with and without PCVCs or PPCs were compared with the Chi-square test for categorical variables (expressed in percentage) and the Student’s t-test (normal distribution) or Wilcoxon rank test (non-Gaussian distribution) for continuous variables (expressed as mean ± standard deviation). Variables with a univariate P<0.15 and those judged clinically important were selected for inclusion in a logistic regression model by stepwise forward selection. Only one variable in a set of variables with a correlation coefficient >0.5 was retained to avoid multicollinearity. Independent predictors of PCVC and PPCs as well as factor-adjusted odds ratios (ORs) with 95% confidence interval (CI) were calculated. Discrimination of the model was assessed with the area under receiver operating characteristic (ROC) and calibration of the model was evaluated with the Hosmer-Lemeshow goodness-of-fit test. For survival analysis, we used a Cox proportional hazards regression model including all risk factors and computed hazard ratios (HR) and 95% CI. Survival curves were computed according to the Kaplan–Meier method, and differences in survival were compared with the log-rank test. All analyses were performed using STATA 14 software (Stata Corp., College Station, TX, USA) and statistical significance was specified as a two-tailed Type I error (P value) set below 0.05.
Results
Between October 1, 2011 and October 31, 2014, 189 patients were screened, 164 provided consent, and 151 were analyzed and followed over a median time of 29 months (range, 2–48 months) after surgery. Thirteen patients were excluded from the study due to patient’s withdrawal (N=8) and cancellation of the planned surgery (N=5). Thirty-day mortality rate was 2.6% and was related to early massive postoperative bleeding (N=1), multi-organ failure in the context of sepsis (N=1) and acute mesenteric ischemia (N=1), and delayed unexplained cardio-respiratory arrest occurring after patient discharge (N=1). The incidence of PCVCs and PPCs was 15% and 33%, respectively. Supra-ventricular arrhythmias were the most common PCVCs (12.6%) whereas atelectasis (24.5%) and pneumonia (15.2%) were the most frequent PPCs followed by prolonged mechanical ventilation (8.6%).
Patients with PCVCs had a higher prevalence of hypertension and alcohol habits, they were more likely to undergo a pneumonectomy and to receive blood transfusion, vasopressors and larger intravenous volumes of crystalloids, compared with patients without PCVCs (Tables 1-3). Patients with PPCs presented a greater prevalence of COPD, hypertension, diabetes mellitus and current smoking; they also presented lower physical performances during CPET and 6MWT than patients without PPCs. In addition, intraoperative vasopressors and blood transfusion were more frequently given in patients with PPCs (Tables 1-3).
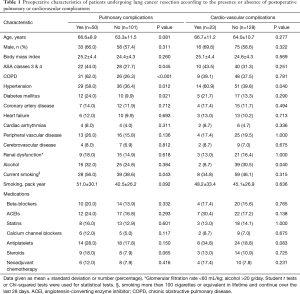
Full table
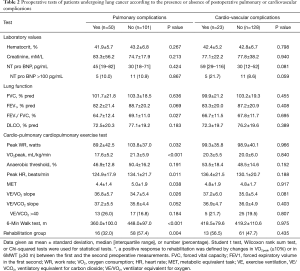
Full table
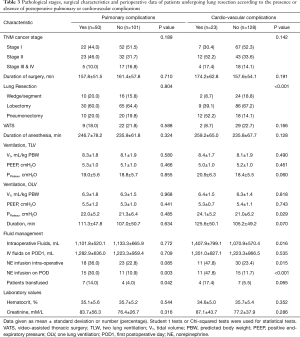
Full table
Multivariate stepwise logistic regression analysis revealed that, elevated plasma levels of NT-pro-BNP (>100 pg/mL) and performance of a pneumonectomy were independent predictors of PCVCs (OR =6.0; 95% CI, 1.3–27.3; OR =9.6; 95% CI, 2.9–31.5, respectively). Likewise, independent risk factors for PPCs included COPD (OR =5.9; 95% CI, 2.4–4.8), current smoking (OR =2.6; 95% CI, 1.1–6.5) and the need for blood transfusion (OR =5.2; 95% CI, 1.2–23.3). In contrast, preoperative rehabilitation was considered a protective factor regarding PPCs (OR =0.13; 95% CI, 0.05–0.34). The area under the curve of the receiver operating curves were 0.85 for PCVCs and PPCs with P values for the Hosmer-Lemeshow goodness of fit (0.814 and 0.685) indicating good discrimination and calibration.
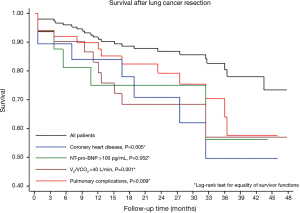
Cox proportional hazards regression analysis and Kaplan-Meyer curves showed that VE/VCO2 slope >40, coronary artery disease, elevated plasma levels of NT-pro-BNP and PPCs were associated with poor survival after surgery (Table 4 and Figure 1).
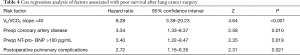
Full table
Discussion
The main findings of this study are summarized as follows: (I) preoperative NT-pro-BNP plasma levels and pneumonectomy were predictive of PCVCs; (II) COPD, current smoking, need for blood transfusion were independent risk factors for PPCs whereas preoperative rehabilitation afforded pulmonary protection; (III) poor mid-term survival was associated with cardiac disease, inefficient ventilation and the occurrence of PPCs.
The prognostic relevance of aerobic capacity and any frailty markers is now well established in elderly and particularly in patients with cancer and chronic diseases (5,15). Besides major clinical factors such as COPD and coronary artery disease, CPET parameters (e.g., VO2peak, anaerobic threshold, VE/VCO2 or end-tidal PCO2) have been shown predictive of early cardio-pulmonary complications and poor survival after thoracic surgery (16-18). In most of these reports, the analysis was focused on a limited number of potential risk factors (e.g., cardio-pulmonary diseases, extent of resection, pathologic stages, PFT, CPET) and was focused either on PPCs or a composite of cardiopulmonary complications that were often poorly defined. In the current study, we collected more than 200 individual data to describe the full spectrum of preoperative patient condition, medical and surgical interventions as well as the time course of postoperative recovery using objective definition criteria for PPCs, PCVCs and other adverse events. Standardized perioperative processes of care entailed preoperative patient selection and optimization coupled with the implementation of fast-tracking interventions involving restrictive fluid administration, mechanical ventilation with low tidal volume and limited inspiratory driving pressure as well as multimodal analgesia and early mobilization.
Pneumonectomy and brain natriuretic peptide were identified as risk factors of PCVCs that mainly consisted in supra-ventricular arrhythmias. Performing extensive lung resections inevitably causes injuries of autonomic nerves and partial remodeling of the left atrium that have been incriminated in the development of arrhythmias (19). Interestingly, increased NT-pro-BNP was found predictive of PCVCs and poor survival. Previous studies have shown that brain natriuretic peptides were valuable predictors of cardiovascular complications, namely atrial fibrillation after thoracic surgery (20). As the release of natriuretic peptides from the cardiomyocytes correlates with the extent of ventricular dysfunction, elevated circulating BNP or NT-pro-BNP have also emerged as predictors of survival after cardiac and non-cardiac surgical procedures (21). Not surprisingly, preexisting coronary artery disease was found predictive survival since ischemic heart diseases remains the first cause of death among Western populations (22).
The occurrence of PPCs was related to known risk factors, namely preexisting COPD and current smoking (4,23). Blood transfusion that was given in a minority of patients (14%) was also predictive of PPCs as it has already been reported elsewhere (24). In the context of frequent postoperative atelectasis, bacterial growth and emergence of pneumonia are promoted by transfusion-induced immunosuppression that is related to reduced natural killer cell activity, decreased interleukin-2 production, lower CD4/CD8 ratio and decreased macrophage activity (25). In contrast with earlier reports, none of the CPET-derived parameters (e.g., VO2peak, anaerobic threshold, VE/VCO2, work load) were identified as a risk factor for PPCs by multivariate analysis. The short preoperative rehabilitation program resulted in improved aerobic fitness (+15% VO2peak) and was associated with a 40% reduction in PPCs. Besides physical training-induced enhancement in physiological reserves, minimally invasive surgical approach and intraoperative patient optimization with hemodynamic control, fluid therapy and ventilatory management likely contributed to improve perioperative outcome, particularly in the higher risk patients (26).
Importantly, mid-term survival after curative lung cancer resection was mostly related to baseline ventilatory efficiency (VE/VCO2 ratio) and the occurrence of PPCs. Calculation of VE/VCO2 ratio (or slope) at the ventilation threshold overcomes the challenging condition of peakVO2 measurements during maximal exercise performance. Nowadays, there is growing scientific evidence of the utility of VE/VCO2 in assessing athletic performances and in providing diagnostic and prognostic information in patients with cardiovascular, pulmonary and neuromuscular diseases as well as in those with deconditioning related to immobilization, sedentarity and aging (11). The VE/VCO2 ratio is independent of peak VO2 as a prognostic marker, and a subgroup of patients with a high VE/VCO2 and a low peak VO2 have been identified to experience the worst outcome (27). Likewise, in a small cohort of 55 COPD patients undergoing lung cancer resection, Shafiek et al. reported that VE/VCO2 slope >35 was a predictor of one-year survival (10). Furthermore, in a larger cohort of 225 candidates for curative lung cancer surgery, Brunelli et al. found that VE/VCO2 slope was the strongest predictor of PPCs, but no data were provided regarding long term outcome (17). From a physiological standpoint, inefficient ventilation during intense exercise reflects ventilation/perfusion mismatch with inappropriate gas exchange that requires higher minute ventilation volume for a given level of CO2 production. As seen from the modified alveolar equation [VE/VCO2 = 863/(1 − VD/VT) × PaCO2; VD, dead space volume; VT, tidal volume], a low PaCO2 setpoint with impaired chemoreflex control and increasing dead space fraction characterizes a pattern of wasted ventilation that contributes to an “exhausting” ventilatory response during exercise as a result of airflow limitations, increasing pulmonary arterial pressure and/or impaired cardiovascular responses (11).
In agreement with recent studies, we found that patients who experienced PPCs were more likely to die after being discharged from the hospital (28-30). We included PPCs based on TNM classification (grade 2 and higher) that ranged from atelectasis requiring supplementary oxygen to respiratory failure requiring ventilatory mechanical support (13). The fact that these minor-to-moderate PPCs were associated with a 2.6-fold increase in mortality is an important message. From a prospective database including 675 patients, Nojiri et al. demonstrated that the occurrence of major PPCs after NSCLC resection was predictive of cancer recurrence (28). Both surgical trauma and bacterial local proliferation in the lungs have been associated with immunosuppression and upregulation of vascular adhesion molecules and that could promote the attachment of residual cancer cells to the endothelium and later tumor recurrence (31). In contrast to PPCs, the occurrence of CVCs did not affect survival in our study owing to their predominantly benign nature (supra-ventricular arrhythmias).
This study had several limitations. First, the study population included patients with early cancer disease stages considered operable according to previously validated criteria. Different risk factors could influence the outcome of patients with more advanced cancer stages and those with greater impairment in physical fitness. Second, the VE/VCO2 ratio was calculated at the respiratory compensation point. Measurements of end-expiratory PCO2 and other methods of the ventilatory equivalent for CO2 (ratio or slope of VE/VCO2) could have provided further prognostic information. In patients with heart failure, determination of VE/VCO2 slope at peak exercise was shown the best predictor of later survival (32). Third, the cause of mortality and information on the progression (or cure) of the cancer was not reported after hospital discharge and we could not assess the effect of PPCs on cancer disease-free survival nor the importance of cardiovascular disease.
In conclusion, this study emphasizes the importance of preexisting cardiopulmonary disease, smoking and the extent of lung resection as risk factors of major complications and poor survival after thoracic surgery. Further studies should be focused on modifiable risk factors to reduce the incidence of PPCs and improve long term outcome. Besides individualized perioperative patient care and fast tracking pathways, patient’s physiological reserves could potentially be enhanced by tobacco withdrawal and adhesion to rehabilitation programs promoting a shift from sedentary to a physically active phenotype.
Acknowledgements
This study was supported by the APSI Funds of the University Hospital of Geneva.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics committee of the University Hospital of Geneva (protocol number: 06–225). Written informed consent was obtained from all participating patients.
References
- Cao B, Bray F, Beltran-Sanchez H, et al. Benchmarking life expectancy and cancer mortality: global comparison with cardiovascular disease 1981-2010. BMJ 2017;357:j2765. [Crossref] [PubMed]
- Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631-9. [Crossref] [PubMed]
- Thomas DC, Blasberg JD, Arnold BN, et al. Validating the Thoracic Revised Cardiac Risk Index Following Lung Resection. Ann Thorac Surg 2017;104:389-94. [Crossref] [PubMed]
- Licker M, de Perrot M, Hohn L, et al. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg 1999;15:314-9. [Crossref] [PubMed]
- Richardson K, Levett DZH, Jack S, et al. Fit for surgery? Perspectives on preoperative exercise testing and training. Br J Anaesth 2017;119:i34-i43. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181-4. [Crossref] [PubMed]
- Brunelli A, Belardinelli R, Refai M, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest 2009;135:1260-7. [Crossref] [PubMed]
- Licker M, Schnyder JM, Frey JG, et al. Impact of aerobic exercise capacity and procedure-related factors in lung cancer surgery. Eur Respir J 2011;37:1189-98. [Crossref] [PubMed]
- Brunelli A. Ventilatory efficiency slope: an additional prognosticator after lung cancer surgery. Eur J Cardiothorac Surg 2016;50:780-1. [Crossref] [PubMed]
- Shafiek H, Valera JL, Togores B, et al. Risk of postoperative complications in chronic obstructive lung diseases patients considered fit for lung cancer surgery: beyond oxygen consumption. Eur J Cardiothorac Surg 2016;50:772-9. [Crossref] [PubMed]
- Weatherald J, Sattler C, Garcia G, et al. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J 2018.51. [PubMed]
- Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41. [Crossref] [PubMed]
- Licker M, Karenovics W, Diaper J, et al. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J Thorac Oncol 2017;12:323-33. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024-35. [Crossref] [PubMed]
- Torchio R, Guglielmo M, Giardino R, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg 2010;38:14-9. [Crossref] [PubMed]
- Brunelli A, Belardinelli R, Pompili C, et al. Minute ventilation-to-carbon dioxide output (VE/VCO2) slope is the strongest predictor of respiratory complications and death after pulmonary resection. Ann Thorac Surg 2012;93:1802-6. [Crossref] [PubMed]
- Brat K, Tothova Z, Merta Z, et al. Resting End-Tidal Carbon Dioxide Predicts Respiratory Complications in Patients Undergoing Thoracic Surgical Procedures. Ann Thorac Surg 2016;102:1725-30. [Crossref] [PubMed]
- Onaitis M, D'Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:368-74. [Crossref] [PubMed]
- Toufektzian L, Zisis C, Balaka C, et al. Effectiveness of brain natriuretic peptide in predicting postoperative atrial fibrillation in patients undergoing non-cardiac thoracic surgery. Interact Cardiovasc Thorac Surg 2015;20:654-7. [Crossref] [PubMed]
- Payne CJ, Gibson SC, Bryce G, et al. B-type natriuretic peptide predicts long-term survival after major non-cardiac surgery. Br J Anaesth 2011;107:144-9. [Crossref] [PubMed]
- Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667-78. [Crossref] [PubMed]
- Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg 2013;148:755-62. [Crossref] [PubMed]
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317-26. [Crossref] [PubMed]
- van Kaam AH, Lachmann RA, Herting E, et al. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med 2004;169:1046-53. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Brunelli A. Preoperative functional workup for patients with advanced lung cancer. J Thorac Dis 2016;8:S840-S8. [Crossref] [PubMed]
- Nojiri T, Hamasaki T, Inoue M, et al. Long-Term Impact of Postoperative Complications on Cancer Recurrence Following Lung Cancer Surgery. Ann Surg Oncol 2017;24:1135-42. [Crossref] [PubMed]
- Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171-6. [Crossref] [PubMed]
- Wang S, Li X, Li Y, et al. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis 2017;9:5143-52. [Crossref] [PubMed]
- Menges P, Kessler W, Kloecker C, et al. Surgical trauma and postoperative immune dysfunction. Eur Surg Res 2012;48:180-6. [Crossref] [PubMed]
- Bard RL, Gillespie BW, Clarke NS, et al. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant 2006;25:589-95. [Crossref] [PubMed]


