Pulmonary function assessment in the early phase of patients with smoke inhalation injury from fire
Introduction
Fire smoke contains superheated gases, steam, and numerous noxious chemical compounds produced by incomplete combustion. It is generally accepted that the most striking features in the airways of the lungs after smoke inhalation are airway obstruction and bronchoconstriction due to intense inflammatory reaction and inspissated secretions caused by inhalation of particulate debris, irritant materials, fumes, or various toxic vapors (1). The survivors from fire accidents may inhale toxic combustion products generated by fires and present with symptoms that resemble asthma, such as severe cough, sputum, choking sensation, and wheezing (2-4).
Bronchial provocation tests (BPTs) are widely used to identify individuals with bronchial hyperresponsiveness (BHR), which is an important pathophysiological feature of asthma (5). The airways of asthmatic patients are narrowed in response to the inhalation of aerosols of sodium chloride (6). The mechanism causing airway narrowing by hypertonic saline is thought to involve an increase in osmolarity and release of mediators from the mast cells and sensory nerves (6,7). Mannitol with high osmolarity is known to be more potent than sodium chloride in producing mast-cell histamines. An increased airway reactivity in previous healthy individuals may be expected after acute exposure to fire smoke, although the studies have shown that pulmonary function abnormalities in burn patients depend on the type of injury (8,9).
The present study was aimed to assess pulmonary functions by using pulmonary function test (PFT) and mannitol BPT in the early phase of patients who had smoke inhalation injury from fires.
Patients and methods
Study design and subjects
The study was approved by the local institutional review board, and the patients permitted their medical records for research from the hospital database. Between January 2010 and January 2012, we conducted an observational study in consecutive patients hospitalized at the burn center of Hallym University Hangang Sacred Heart Hospital in Seoul, South Korea.
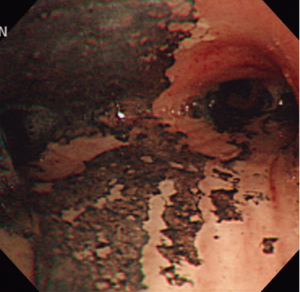
The inclusion criteria were as follows: adult age, minor burns involving a total body surface area of less than 15%, initial arterial carboxyhemoglobin (COHb) levels of more than 5%, or a history of exposure to smoke in closed space and presence of singed nasal hairs, sooty sputum, or auscultatory findings such as wheezing or rhonchi. Diagnosis of inhalation injury was made by fiberoptic bronchoscopy, which was normally performed within 48 h after admission. We characterized bronchoscopic findings for inhalation injury as follows: edema, blistering, carbonaceous material, soot, hemorrhage, inflammation, ulceration, and necrosis of the bronchial mucosa (Figure 1). The severity of inhalation injury was graded according to the number of presenting features: near normal, no features present; mild, 1-3 features present; moderate, 4-6 features present; severe, 7-8 features present (10). Patients who had underlying structural lung diseases and past history of tuberculosis, asthma, and atopy were excluded. After admission, patients with smoke inhalation were treated according to the lung care standards of our burn unit, including fluid and nutritional support, maneuvers to prevent pneumonia, endotracheal intubation for airway maintenance, and mechanical ventilation if indicated. We also performed high-resolution computed tomography (HRCT) to identify lung parenchymal involvement of inhalation injury.
During the study periods, the subjects complaining of cough were recruited after matching for age, sex, height and body mass index from our outpatient department after obtaining written informed consent. All participants who had suffered from cough for more than three weeks but had no previous history of respiratory disease or atopy.
High-resolution computed tomography (HRCT)
Computed tomography (CT) scans were acquired using a GE HiSpeed CT/i scanner (GE Medical System, Milwaukee, WI, USA) with no use of contrast media. All CT data were reconstructed by using a high-spatial-frequency (bone) algorithm with a section thickness of 1 mm. Window width and window levels were 400 HU and 20 HU for the mediastinum, and 1,500 HU and –700 HU for the lungs, respectively.
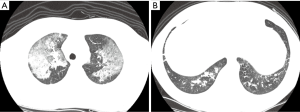
If HRCT findings showed peribronchial ground glass opacity with/or without consolidation, bronchial wall thickenings, branching linear attenuations, atelectasis, or interlobular septal thickening in patients with fire smoke inhalation, we considered inhalation injury to have resulted in lung parenchymal involvement (Figure 2).
Each CT finding was interpreted by a radiologist, and defined as; ground glass opacity, a hazy area of air space of increased lung attenuation with preservation of bronchial and vascular markings; consolidation, alveolar space that contains liquid instead of gas; atelectasis, a condition where the alveoli deflated; branching linear attenuations referred to as poorly defined, small centrilobular nodules and branching linear opacity of small airways (2-4 mm diameter) originate from a single stalk; bronchial wall thickening, greater than 0.3 mm or half the width of the accompanying pulmonary artery branch; interlobular septal thickening, greater than 2.0 cm in length and 0.1 mm in thickness (11,12).
PFT and mannitol bronchoprovocation test
Before the PFT and the mannitol BPT, all subjects were asked to refrain from taking short-acting bronchodilators for 6 hours and long-acting bronchodilator for 12 h prior to the study. And the two tests were performed on separate days.
For pulmonary functions test, we used a spirometer (Vmax22; SensorMedics; Yorba Linda, CA, USA), which was calibrated with a 3 L syringe on the morning of each day. In accordance with the American Thoracic Society, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), forced expiratory flow between 25% and 75% of the FVC (FEF25–75), peak expiratory flow (PEF) and diffusing capacity of the lung to transfer carbon monoxide (DLco) were measured. Subsequently, post-bronchodilator values were measured 15-20 min after the administration of an inhaled bronchodilator (e.g., 400 μg of salbutamol) (13). Bronchodilator response (BDR) was assessed by comparing pre- and post- bronchodilator FEV1. An increase in FEV1 greater than 200 mL and 12% of the baseline value was accepted as a positive BDR (14).
For mannitol BPT, dry powder mannitol (ARIDOLTM) was prepared in kit form (Pharmaxis Ltd., NSW Australia) containing several capsules with different doses of mannitol. A single capsule contained 0, 5, 10, 20, or 40 mg. The dry powder device used for inhalation was the OsmohalerTM (RS-01, PastiapeTM, Italy). Baseline FEV1 was measured on presentation at our PFT laboratory. The dose protocol consisted of 0 (empty capsule as a placebo), 5, 10, 20, 40, 80, 160, 160 and 160 mg (15). Doses of more than 80 mg were given in multiples of 40 mg capsules. The FEV1 was measured twice 60 s after each dose, and the highest FEV1 value was recorded. The FEV1 value measured after the 0-mg capsule was taken as the pre-challenge FEV1 and was used to calculate the percentage decrease in FEV1 in response to the mannitol challenge. If the FEV1 fell by more than 10% in response to a single dose, the same dose was repeated. The mannitol challenge was completed when a 15% fall in FEV1 was documented or a cumulative dose of 635 mg had been administered. After the completion of the test, an inhaled bronchodilator (e.g., 200 μg of salbutamol) was administered.
Statistical analysis
Data were analyzed using dBSTAT for Windows Version 4.0 (DBSTAT Co, Chuncheon, South Korea). Categorical variables were described as frequencies (%) and continuous variables as mean ± standard deviation. We conducted independent two-sample and paired t-tests for the comparison of continuous variables and the χ2 test or Fisher’s exact test (if the expected values were below 5) for categorical variables. A P value of less than 0.05 was considered statistically significant.
Results
Subjects characteristics
In all, 30 adult participants were enrolled in this study: 15 in fire smoke group (5 men and 10 women) and chronic cough group in 15 (5 men and 10 women).
In fire smoke group, the majority of the patients (86.7%) suffered only from smoke inhalation; only 2 of 15 patients required E-tube insertion for airway maintenance and were admitted to the intensive care units. Initial arterial COHb levels and the PaO2/FiO2 ratio were 14.81%±18.40% and 425.68±123.69 mmHg, respectively. Bronchoscopy was performed in all patients, and the grades for inhalation injury were as follows bronchoscopically: near normal, 3 (20%); mild, 9 (60%); moderate, 3 (20%); severe, 0. HRCT was performed in seven patients, and four of those (57.1%) showed the findings compatible with lung parenchymal involvement of inhalation injury (Table 1).
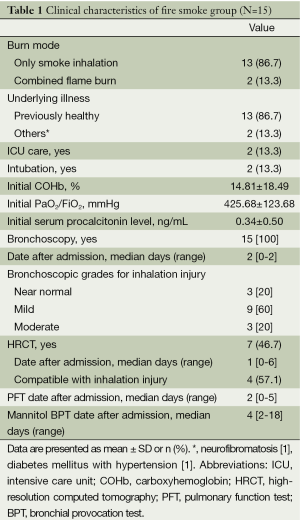
Full table
There were no significant differences in demographics and clinical symptoms between the two groups. The most common symptom was cough, 46.7% and 73.3% in fire smoke and chronic cough group, respectively (Table 2).
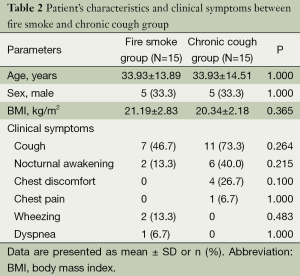
Full table
HRCT findings
Of the seven patients in whom HRCT was performed, 4 (57.1%) were found to have lung parenchymal abnormalities on HRCT scans resulted from fire smoke inhalation injury. Peribronchial ground glass opacities were the most common, and followed by segmental or subsegmental consolidations, atelectasis, branching linear attenuations, bronchial wall thickening, and interlobular septal thickening (Table 3).
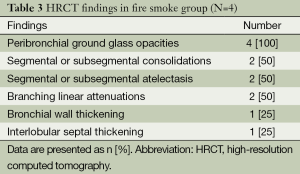
Full table
PFT and mannitol bronchoprovocation test
In fire smoke group, FVC and FEF25-75 were slightly less than 80% predicted, and other values including FEV1, PEF, FEV1/FVC and DLco were equal to or a little more than 80% predicted. Then, the values of FEF25-75 and the percent of FEF25-75 before and BDR were 2.4±1.47 L vs. 2.65±1.45 L (P=0.045), and 68.7±37.29% vs. 76.4±36.70% (P=0.031), respectively. The positive BDR was observed in two patients (13.3%) (Table 4).
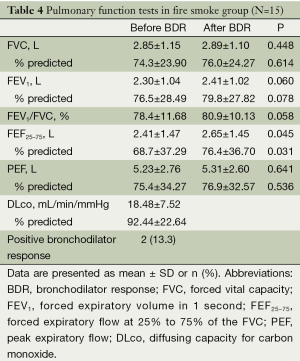
Full table
We compared the results of mannitol BPTs in the two groups, but found no significant differences in baseline FEV1, maximum decrease in FEV1, and the total cumulative mannitol dose at the time of the maximum decrease in FEV1. Moreover, all the subjects showed mannitol BPTs within normal limits (Table 5). All study participants were reported to tolerate the tests well without any side effects except complaining of mild cough.

Full table
Discussion
The fire victims suffering from smoke inhalation may have a number of complications caused by various pulmonary sequelae. Wheezing and chronic cough may reflect underlying hyperreactive airways. Chronic bronchitis, bronchiolitis obliterans, bronchial stenosis, lung fibrosis, bronchiectasis and atelectasis have been reported to be resulted from exposure to fire smoke and subsequent inflammatory consequences (16).
The understanding of precise pathophysiology in patients who had smoke inhalation damage from fires is of great important for effective airway management. The present study aimed to assess the lung functions in the early phase of patients with smoke inhalation injury. Initially, we taken into account that fire smoke inhalation is a strong stimulus that triggers bronchoconstriction. Unfortunately, we could not detect any significant BHR in mannitol BPTs to explain airway narrowing that commonly occurs after fire smoke inhalation (2,3). Although FEV1, PEF, FEV1/FVC and DLco were equal to or a little more than 80% predicted, post-bronchodilator FVC and FEF25-75 were less than 80% predicted. Moreover, the percent of FEF25-75 was significantly increased after BDR compared to the value before BDR, 68.7% to 76.4% (P=0.031). This finding gives us a relevance to initiate bronchodilators for airway management in patients who had smoke inhalation injury from fires.
Currently, there are two categories of BPTs defined by the mechanisms whereby they stimulate the airways to narrow. The direct stimuli include pharmacologic agents such as histamine or methacholine, which act directly on specific receptors on the bronchial smooth muscle to cause airway contraction. The indirect stimuli refer to physical stimuli such as exercise; hyperventilation; osmotic aerosols including hypertonic saline or mannitol; and adenosine monophosphate, which trigger airway narrowing indirectly by causing the release of various contractile mediators (e.g., leukotrienes, prostaglandins, and histamine) from inflammatory cells within the airways (15). The ultimate goal of precise evaluation of airway narrowing due to an inhalation injury is to provide optimal early management (17-20).
After fire smoke inhalation, a variety of consequences in the respiratory system may be followed by an intense cellular response with neutrophil infiltration, airways edema, bronchoconstriction from aerosolized irritants, small airway occlusion from sloughed endobronchial debris or cast, and alveolar flooding from epithelial disruption (21). These injuries evolve over time and parenchymal lung dysfunction is often minimal for 24-72 h after fire smoke inhalation (22). The airways obstruction and bronchocontriction, which mimic asthma, and usually manifest themselves in the first 24 h (23). In the previous studies, the airways reactivity was assessed by mainly methacholine test, which showed often positive results (2,3). Then, we applied mannitol BPTs on the median four days after admission to a narrow spectrum of patients who mostly had smoke inhalation. Kinsella et al. reported that BHR to methacholine usually manifested itself within three days of injury (2). In our study, it remains unclear why BHR to inhaled mannitol was not produced. A delay of the BPT may be a factor not to provoke BHR in fire smoke group. In addition to, one possible explanation is that the airway epithelium after smoke inhalation may be severely injured or even denuded to a degree so that the changes in the airway osmolarity in response to inhaled mannitol could not have occurred. Another reason is that the pathological findings in smoke inhalation injury are quite different from those in asthma, that is, chronic bronchitis with eosinophil infiltration. We have experienced airway inflammatory cells composed mainly of neutrophils, not eosinophils, in the acute stage of inhalation injury (Figure 3) (24). Likewise, this is why the study participants did not have BHR in mannitol BPTs, even if they had asthma-like symptoms. Moreover, the increased airway reactivity and prolonged obstructive airway disease have been reported to persist from 3 to 6 months as long-term sequelae of smoke inhalation injury (2,3,25). These findings are the cases when fire victims accompanying inhalation injury suffered from major burn, which probably might have resulted into prolonged BHR, due to intense systemic inflammatory reaction. In our study, the majority of patients had smoke inhalation injury and not fire-related injury. This fact would have contributed to their lack of BHR.
Chest CT scans have been reported to show the superior sensitivity in comparison to chest radiographs for the detection of pulmonary lesion in the early hours of inhalation injury (26). Yamamura et al. reported bronchial wall thickening, greater than 2 mm at initial CT scan, was a predictive marker of prolonged intensive care unit (ICU) stays and the development of pneumonia in patients with inhalation injury (27). In our study, although CT findings such as ground glass opacity with/or without consolidation, atelectasis, branching linear attenuation and interlobular septal thickening, were obtained in patients with smoke inhalation, however, it remains as to which findings affect the pulmonary consequences and how much respiratory function restores since the injury.
The limitations of our study include a small sample size as well as the fact that the participants with chronic cough did not represent individuals with no exposure to fire smoke in whom PFTs were not routinely performed. In addition, we did not have long-term data on late sequelae of inhalation injury because some of the patients refused to undergo BPTs several months after discharge because they did not complain of further symptoms. Despite these limitations, this study demonstrates that FEF25-75, reflecting obstructive small airway disease pattern, was mild decreased before BDR, and significant increased after BDR in fire smoke group, and a small number of patients had positive BDR. These findings may indicate a potential benefit from the use of bronchodilator drugs (28).
In conclusion, the present preliminary study shows that fire smoke inhalation has something with mild obstructive small airway disease pattern of pulmonary function in the early phase in patients with inhalation injury. However, more evidence is necessary to know as to the relationship between airway narrowing to inhaled mannitol and smoke inhalation injury through a large-scaled prospective study.
Acknowledgements
The authors would like to thank the volunteers who agreed to participate in this study.
Disclosure: The authors declare no conflict of interest.
References
- Thorning DR, Howard ML, Hudson LD, et al. Pulmonary responses to smoke inhalation: morphologic changes in rabbits exposed to pine wood smoke. Hum Pathol 1982;13:355-64. [PubMed]
- Kinsella J, Carter R, Reid WH, et al. Increased airways reactivity after smoke inhalation. Lancet 1991;337:595-7. [PubMed]
- Park GY, Park JW, Jeong DH, et al. Prolonged airway and systemic inflammatory reactions after smoke inhalation. Chest 2003;123:475-80. [PubMed]
- Pruitt BA Jr, Flemma RJ, DiVincenti FC, et al. Pulmonary complications in burn patients. A comparative study of 697 patients. J Thorac Cardiovasc Surg 1970;59:7-20. [PubMed]
- Sterk PJ, Fabbri LM, Quanjer PH, et al. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:53-83. [PubMed]
- Schoeffel RE, Anderson SD, Altounyan RE. Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. Br Med J (Clin Res Ed) 1981;283:1285-7. [PubMed]
- Anderson SD, Smith CM. Osmotic challenges in the assessment of bronchial hyperresponsiveness. Am Rev Respir Dis 1991;143:S43-6. [PubMed]
- Whitener DR, Whitener LM, Robertson KJ, et al. Pulmonary function measurements in patients with thermal injury and smoke inhalation. Am Rev Respir Dis 1980;122:731-9. [PubMed]
- Demling RH, Crawford G, Lind L, et al. Restrictive pulmonary dysfunction caused by the grafted chest and abdominal burn. Crit Care Med 1988;16:743-7. [PubMed]
- Khoo AK, Lee ST, Poh WT. Tracheobronchial cytology in inhalation injury. J Trauma 1997;42:81-5. [PubMed]
- Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327-31. [PubMed]
- Rossi SE, Franquet T, Volpacchio M, et al. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2005;25:789-801. [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [PubMed]
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- Anderson SD, Brannan J, Spring J, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med 1997;156:758-65. [PubMed]
- Lee-Chiong TL Jr. Smoke inhalation injury. Postgrad Med 1999;105:55-62. [PubMed]
- Brannan JD, Anderson SD, Freed R, et al. Nedocromil sodium inhibits responsiveness to inhaled mannitol in asthmatic subjects. Am J Respir Crit Care Med 2000;161:2096-9. [PubMed]
- Brannan JD, Anderson SD, Gomes K, et al. Fexofenadine decreases sensitivity to and montelukast improves recovery from inhaled mannitol. Am J Respir Crit Care Med 2001;163:1420-5. [PubMed]
- Brannan JD, Gulliksson M, Anderson SD, et al. Inhibition of mast cell PGD2 release protects against mannitol-induced airway narrowing. Eur Respir J 2006;27:944-50. [PubMed]
- Brannan JD, Koskela H, Anderson SD, et al. Budesonide reduces sensitivity and reactivity to inhaled mannitol in asthmatic subjects. Respirology 2002;7:37-44. [PubMed]
- Walker HL, McLeod CG Jr, McManus WF. Experimental inhalation injury in the goat. J Trauma 1981;21:962-4. [PubMed]
- Pruitt BA Jr, Erickson DR, Morris A. Progressive pulmonary insufficiency and other pulmonary complications of thermal injury. J Trauma 1975;15:369-79. [PubMed]
- Sheridan RL. Airway management and respiratory care of the burn patient. Int Anesthesiol Clin 2000;38:129-45. [PubMed]
- Clark CJ, Pollock AJ, Reid WH, et al. Role of pulmonary alveolar macrophage activation in acute lung injury after burns and smoke inhalation. Lancet 1988;2:872-4. [PubMed]
- Moisan TC. Prolonged asthma after smoke inhalation: a report of three cases and a review of previous reports. J Occup Med 1991;33:458-61. [PubMed]
- Reske A, Bak Z, Samuelsson A, et al. Computed tomography--a possible aid in the diagnosis of smoke inhalation injury? Acta Anaesthesiol Scand 2005;49:257-60. [PubMed]
- Yamamura H, Kaga S, Kaneda K, et al. Chest computed tomography performed on admission helps predict the severity of smoke-inhalation injury. Crit Care 2013;17:R95. [PubMed]
- Kim JY, Kim CH, Shin HW, et al. The Findings of Pulmonary Function Test in Patients with Inhalation Injury (Korean). Tuberc Respir Dis 2006;60:653-62.


