
Five-year outcomes after thoracic endovascular aortic repair of symptomatic type B penetrating aortic ulcer with intramural hematoma in Chinese patients
Introduction
Acute aortic syndromes (AASs) consist of 3 interrelated conditions with similar clinical characteristics, including “classic” aortic dissection (AoD), penetrating aortic ulcer (PAU) and aortic intramural hematoma (IMH). In the American Heart Association guidelines published in 2010, these entities were briefly discussed in the context of three overlapping aortic lesions: intimal defect without IMH, intimal defect with IMH and IMH without an intimal defect. Meanwhile, the course, morbidity and mortality of each treatment remain unknown owing to the lack of large series in the literature. A conservative approach to uncomplicated type B IMH, such as antihypertensive treatment and watchful monitoring, is currently preferred because it appears to be the safety strategy. However, in some cases, the disease may still progress despite optimal medical treatment. Notwithstanding some recommendations for endovascular repair of AAS (1-3), the indications for IMH and PAU remain controversial, and the general approach is to treat them in the same way as AoD. Some authors have asserted that PAU is an entity defined as a focal lesion that ulcerates the intima and disrupts the internal elastic lamina of the aortic wall (4). There are two main etiologies: PAU and ulcer-like projection (ULP) secondary to intimal rupture during IMH evolution. Unfortunately, clinical overlap between PAU and ULP in past decades has provoked confusion regarding the frequency, prognosis and management of these entities. In our study, ulcers had already been found in the first computed tomography angiography (CTA) scan after symptom onset instead of emerging in the follow-up CTA scan during IMH evolution. Therefore, in this article, we still use the term PAU to describe the protrusion of the aorta wall. Here, we aimed to evaluate the feasibility and long-term follow-up results of thoracic endovascular aortic repair (TEVAR) for symptomatic type B PAU associated with IMH in Chinese patients.
Methods
Definitions in this article
Symptomatic: severe, first-time, sudden-onset chest/back pain of a sharp/tearing character.
PAU: lesion with ulceration that penetrates the internal elastic lamina. A mushroom-like lesion with outpouching of the aortic lumen and overhanging edges on CTA.
IMH: crescent-shaped or circular thickening of the aortic wall without contrast enhancement on CTA.
Patients
From January 2009 to April 2013, 118 patients were admitted to our department with a diagnosis of AAS confirmed by urgent CTA examination. All patients complained of severe, somewhat uniform, first-time, sudden-onset chest/back pain of a sharp/tearing character (‘aortic pain’). Thirty cases were diagnosed as Stanford type B PAU with IMH. Two patients were reluctant to consent to further intervention for personal reasons. Twenty-eight patients underwent TEVAR. The patient’s management pathway was showed in Figure 1.
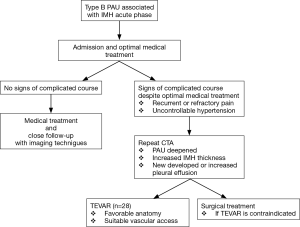
Initial medical stabilization targets included heart rate less than 70 bpm and systolic blood pressure between 100 and 120 mmHg, usually managed by beta-blockers and antihypertensive agents administered orally or intravenously. Pain control therapy was carried out based on routine hospital protocol.
Preoperative imaging evaluation
All 28 cases underwent urgent CTA examination in the emergency department before admission. A contrast-enhanced three-phase multidetector-row CTA of the entire aorta (from the level of the midpoint of the neck to at least 2 cm distal to the bifurcation of the superficial and deep femoral artery) was performed according to the hospital protocol. All images were evaluated at a digital workstation and reconstructed by multiplanar reformation (MPR) and maximum intensity projection (MIP). Detailed measures were documented as recommended (1), including the following: the segment where the first PAU appeared, the segments involved in IMH, the number of PAUs, the diameter of the aorta at the middle aortic arch (between the left common carotid and subclavian arteries), the diameter of the proximal descending thoracic aorta [commencing at the isthmus, approximately 2 cm distal to the left subclavian artery (LSCA)], and the diameter of the middle descending aorta (at the midpoint between the proximal descending thoracic aorta and the aorta at the diaphragm). The maximal thickness of the IMH was also recorded. The diameters on both sides of the common femoral and iliofemoral arteries were documented to evaluate the routes of access for TEVAR. Aortic calcification, pleural effusion and pericardial effusion were noted.
All 28 patients still had recurrent or refractory pain or uncontrollable hypertension despite optimal medical treatment. Then, CTA was repeated according to the same protocol to evaluate any sign of the progression of PAU associated with IMH. The mean delay time from admission to repeated CTA was 13.5 days (7 to 15 days). Then, an urgent/emergency TEVAR procedure was performed.
Surgical technique
All TEVAR procedures were performed in a digital subtraction angiography (DSA) theater under general anesthesia. The femoral artery approach was performed with a small incision in the groin. Angiography and stent graft delivery were conducted according to the standard protocol. The abdominal aorta, thoracic aorta, vertebral artery and complete circle of Willis were evaluated before and after deployment. During stent deployment, systolic blood pressure was controlled at the level of 100 to 120 mmHg. No preemptive cerebrospinal fluid drainage or spinal cord protection was used during TEVAR. Technical success was defined as proper deployment of the graft at the landing zone as preoperationally planned, with total coverage of the lesion and no contrast in the lesion after deployment.
Postoperative care and follow-up
All patients were transferred to the intensive care unit immediately after the endograft procedure. Respiratory assistance was terminated after the patients regained consciousness. A heart rate less than 80 bpm and systolic blood pressure between 100 and 120 mmHg were maintained. No anticoagulant was necessary unless otherwise indicated.
Telephone follow-up was conducted in the first week after discharge, followed by consultation at the outpatient clinic in the 1st month. Then, regular CTA follow-up was conducted by appointment in the 3rd, 6th, 12th, 24th, and 60th months (±1 month) after TEVAR. For subsequent long-term follow-up, telephone interviews and clinical consultation were continued every 6 months.
Results
In-hospital results
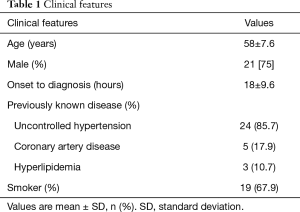
The clinical features of 28 patients are shown in Table 1. There was no in-hospital mortality. Among the 28 cases, 22 (78.6%) complained of periodic attacks of similar pain even with strict blood pressure control, and 5 cases (17.9%) complained of recurrent intolerable lumbar back discomfort. No sign of ischemia was observed in any of the cases. Seven (25%) cases had creatinine levels that increased at least 26.5 µmol/L over baseline during their hospital stay (5).
Full table
The urgent CTA results before admission are shown in Table 2. Most of the patients had PAUs, with a mean of 1.3 [1–5] ulcers. The most likely segment for the first PAU to appear was from the LSCA to the middle descending aorta (25/28, 89%).

Full table
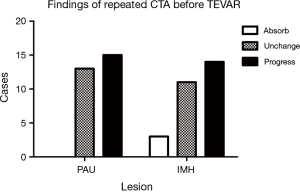
After a mean of 13.5 days of initial medical stabilization, repeated CTA was performed due to symptom recurrence. The results are listed in Figure 2. Notice that no PAU was thrombosed, while IMH showed progression in 50% of cases. We also found that the most absorbed segment was the area near the LSCA; the mean thickness was reduced by 2.1±0.6 mm in this group of patients.
All TEVAR procedures were technically successful. Twenty-nine stent grafts were implanted, including the Talent/Valiant Thoracic Stent Graft (Medtronic, Inc., Northridge, CA, USA), the Hercules Thoracic Stent-Graft System (Shanghai MicroPort Lifesciences Co. Ltd., Shanghai, China), and the Zenith TX2 TAA Endovascular Graft (Cook, Inc., Bloomington, IN, USA). The membrane coverage length ranged from 160 to 215 mm according to the stent product design. A 0-, 2-, 4- or 6-mm taper stent was selectively deployed according to aorta diameter. The most frequently used proximal stent diameter was 32 mm (28–40 mm). One patient needed 2 stents to achieve effective coverage of the PAUs and IMH. The ostium of the LSCA was intentionally completely covered in 3 (11%) cases. Retrograde perfusion though the left vertebral artery was confirmed by angiography immediately after stent deployment. Partial coverage was achieved in 16 cases. No post-dilation or adjuvant procedures were needed in any of the patients.
The average respiratory assistance time was 1.2 hours (0.5–3 hours). The mean ICU stay time was 8 hours (2–23 hours). The mean length of postoperative hospitalization was 6.5 days (5–9 days). There were no symptoms of limb ischemia and no surgical incision-related complications.
Follow-up results
Two patients were lost to follow-up due to relocation at the 4th and 16th months. The follow-up rate was 92.8%. The 1-, 2-, and 5-year overall survival rates were 100%, 100%, and 96.1%, respectively. One patient died after 42 months due to a car accident. In 20 (71%) patients, the typical aortic pain was resolved, and the patients remained asymptomatic. Eight (29%) patients complained of lumbar back discomfort during early follow-up; the longest duration of lumbar discomfort was 7 months. Two heavy smokers in whom the ostium of LSCA was completely covered by the graft had experienced transient dizziness upon resumption of smoking during follow-up. Both of these patients underwent neurological evaluation by a neurologist through a CT scan in the emergency department. No newly occurring brain infarctions or bleeding existed. After strict smoking cessation, similar symptoms of dizziness no longer occurred. No patient needed reintervention. Four patients safely underwent nonvascular surgery. A weakened left radial artery pulse was observed in 6 (22.2%) cases within 6 months, but there was no accompanying pain/paresthesia/paralysis/pallor. Twenty-six patients (93%) achieved grade V of left upper limb muscle strength. Only 2 (7.4%) cases were graded as IV within 6 months, and both improved thereafter. All patients with pleural effusion showed spontaneous absorption at the first CTA follow-up. Most PAUs (26 cases, 93%) were well covered and had completely disappeared by the first follow-up CTA without endoleaks. In 2 (7.7%) cases, CTA showed that the first PAU was absorbed; however, type II endoleak was observed at the distal part of graft due to retrograde intercostal artery blood flow. No aortic expansion was observed on the 60th month CTA scan. Most IMHs (21 cases, 75%) were well absorbed as of the 3rd month. In 6 (22.2%) cases, absorption was observed as of the 6th month. In 9 (34.6%) cases, CTA showed residual PAU at the suprarenal abdominal aorta without IMH at the end of the study. All these patients underwent regular follow-up according to the protocol, and no aneurysm formation or rupture could be found.
Discussion
PAU is now known to have a particularly malignant nature (6). Symptomatic PAU associated with IMH tends to be especially likely to pose a high risk (7). Some authors have described the typical patient as elderly, usually over 65 years of age, with hypertension and diffuse atherosclerosis (8). In our study of Chinese patients, the participants had a mean age of 58 years and were typically male, hypertensive, and active smokers. Hypertension may play a key role in aortic abnormality progression in the Asian population (9). Upon review of all 24 hypertensive patients, they had undergone no regular blood pressure monitoring in at least 3 months before admission. A possible cause might be the lack of effective health education in China. We have also observed that most patients had more than one PAU, and the first PAU usually appeared in the segment from the LSCA to the middle descending aorta (24/28, 89%). The repeated CTA showed that even with optimal medical stabilization, the PAU and IMH still progressed in some way in most patients, manifested as PAU enlargement and deepening as well as IMH thickening after the initial onset.
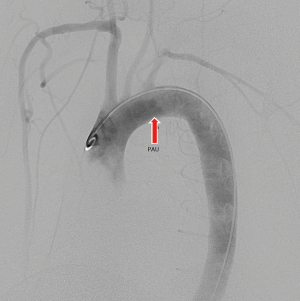
A typical case’s CTA series is shown in Figures 3-6.



Our results may suggest that in Chinese patients with symptomatic PAU associated with IMH, self-healing is unlikely to occur. The shear force to the aortic wall in the segment from LSCA to middle descending aorta was higher than in another part of it, which may be due to the natural curvature (10). The presence of PAU and IMH, which reduce the vessel wall tolerance in this ‘dangerous’ segment, may combine with hypertension/high shear force to cause disastrous consequences, such as dissection/rupture, thereby enhancing the difficulty in treatment and increasing mortality. Symptomatic PAUs associated with IMH were likely to have an increased risk compared with simple asymptomatic PAU or IMH without PAU (11). Awareness should be raised among physicians, and ‘more aggressive’ treatment should be considered (12). TEVAR was an option for these patients because of its minimally invasive nature. In this study, the results of TEVAR for these patients were quite acceptable. The technical success rate was encouraging. The follow-up data also showed a good prognosis for most patients. However, one situation should be noted: the neurological complication seems to have been elevated in our study when the LSCA was completely covered, especially for active smoker, even when the pre- and postprocedural angiography showed a complete circle of Willis and good retrograde left vertebral artery blood flow. One report showed that only 4% of cases had symptoms sufficient to warrant subsequent revascularization (13). The method for LSCA revascularization can include surgical bypass or interventional reconstruction (14). Bifurcated stent grafts for LSCA reconstruction are now officially available on the Chinese market. These devices may be an option for those patients whose LSCA needs to be intentionally covered.
Conclusions
The five-year outcomes of TEVAR for symptomatic Stanford type B penetrating aortic ulcer associated with intramural hematoma in Chinese patients were encouraging. This treatment is a feasible option. Long-term follow-up is necessary.
Limitation
The main limitations of this study are the small number of patients and the short follow-up duration. However, because there are so few Chinese large-series studies in the literature, multicenter studies are anticipated to allow a better understanding of the natural history, epidemiology and long-term results of these particular lesions.
Acknowledgements
Funding: This study was financially supported by grants from the Pudong New District Health and Family Planning Commission, Implementation Plan of Health Science and Technology Projects (PW2015D-2).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of Renji Hospital, Shanghai Jiao Tong University School of Medicine, approved the use of a prospectively maintained database of patients with symptomatic type B acute aortic syndrome who received thoracic endovascular aortic repair for this retrospective study (No. [2015]13K).
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Baikoussis NG, Apostolakis EE, Siminelakis SN, Papadopoulos GS, Goudevenos J. Intramural haematoma of the thoracic aorta: who’s to be alerted the cardiologist or the cardiac surgeon? J Cardiothorac Surg 2009;4:54. [Crossref] [PubMed]
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [Crossref] [PubMed]
- Vilacosta I, Aragoncillo P, Canadas V, et al. Acute aortic syndrome: a new look at an old conundrum. Heart 2009;95:1130-9. [PubMed]
- Howitt SH, Grant SW, Caiado C, et al. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: a validation study. BMC Nephrol 2018;19:149. [Crossref] [PubMed]
- Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg 2012;55:10-5. [Crossref] [PubMed]
- Ganaha F. Prognosis of Aortic Intramural Hematoma With and Without Penetrating Atherosclerotic Ulcer: A Clinical and Radiological Analysis. Circulation 2002;106:342-8. [Crossref] [PubMed]
- Bischoff MS, Geisbusch P, Peters AS, et al. Penetrating aortic ulcer: defining risks and therapeutic strategies. Herz 2011;36:498-504. [Crossref] [PubMed]
- Cho JR, Shin S, Kim JS, et al. Clinical characteristics of acute aortic syndrome in korean patients: from the korean multi-center registry of acute aortic syndrome. Korean Circ J 2012;42:528-37. [Crossref] [PubMed]
- Taguchi E, Nishigami K, Miyamoto S, et al. Impact of shear stress and atherosclerosis on entrance-tear formation in patients with acute aortic syndromes. Heart Vessels 2014;29:78-82. [Crossref] [PubMed]
- Patel HJ, Williams DM, Upchurch GR Jr, et al. The challenge of associated intramural hematoma with endovascular repair for penetrating ulcers of the descending thoracic aorta. J Vasc Surg 2010;51:829-35. [Crossref] [PubMed]
- Evangelista A, Czerny M, Nienaber C, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg 2015;47:209-17. [Crossref] [PubMed]
- Dunning J, Martin JE, Shennib H, et al. Is it safe to cover the left subclavian artery when placing an endovascular stent in the descending thoracic aorta? Interact Cardiovasc Thorac Surg 2008;7:690-7. [Crossref] [PubMed]
- Al-Hakim R, Schenning R. Advanced Techniques in Thoracic Endovascular Aortic Repair: Chimneys/Periscopes, Fenestrated Endografts, and Branched Devices. Tech Vasc Interv Radiol 2018;21:146-55. [Crossref] [PubMed]


