
Clinical implications of discrepant results between genotypic MTBDRplus and phenotypic Löwenstein-Jensen method for isoniazid or rifampicin drug susceptibility tests in tuberculosis patients
Introduction
Tuberculosis (TB) remains a major cause of infectious diseases leading to mortality and morbidity worldwide. Its control is seriously hampered by the emergence of multidrug-resistant (MDR) TB, which has arisen because of resistance to both isoniazid (INH) and rifampicin (RIF), 2 pivotal drugs in TB treatment. In a global TB report by the World Health Organization (WHO), the incidence of MDR TB was estimated to be about 3.5% of new cases and 18% of previously treated cases (1). Globally in 2017, MDR TB occurred about in 458,000 people and caused 230,000 deaths. In South Korea, 852 cases of MDR TB were reported, which represented 2.2% of all TB patients in 2016 (2). Despite the low percentage of MDR TB relative to the total number of TB cases, a poor response to anti-TB treatment is important in clinical practice because it is more difficult to treat on an individual basis and, consequently the disease may have greater chance of spreading to others.
The conventional drug susceptibility test (DST) for TB is a phenotypic culture-based method that involves detection of the growth of Mycobacterium tuberculosis (M. tuberculosis) when exposed to specific concentrations of individual anti-TB drugs. Although it is considered the gold standard, the test requires a long time for the result and poses a biosafety risk. A study from South Korea reported that it takes about 80 days for a clinician to receive the DST report and to choose a proper regimen for treating TB (3). Recent advances in technology and knowledge about the pathogen have led to the development of molecular DSTs, which can check for mutations conferring resistance to specific anti-TB medications. The commonly used molecular assays endorsed by the WHO are the GenoType®MTBDRplus (Hain Lifescience, Nehren, Germany) and Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) tests (4). The advantages of these molecular DSTs are the faster turnaround time for the result and less biohazard risk than the phenotypic DST.
The MTBDRplus test is a line probe assay that can detect resistance to both INH and RIF. This assay uses probes to mutations in specific regions of katG and inhA for INH-resistant M. tuberculosis and of rpoB for RIF-resistant M. tuberculosis. Whereas the Xpert MTB/RIF test detects only RIF-resistant M. tuberculosis, the MTBDRplus test can detect MDR M. tuberculosis in a faster and safer way than previous tests. Molecular test has good diagnostic performance; for example, the sensitivity and specificity of the MTBDRplus test for the detection of resistance to RIF are 98.1% and 98.7%, respectively. The sensitivity is lower (84.3%) and specificity is higher (99.5%) for INH (5).
With the wide use of molecular assays, clinicians sometimes encounter discrepancies between the results of phenotypic and molecular DSTs for anti-TB drugs. Moreover, the diagnostic accuracy of these molecular methods depends on the frequency of the mutation in various geographic regions (6,7). These factors present a clinical challenge and complicate decision-making about the management of TB patients. Although some expert opinions for addressing this dilemma have been proposed (4,8,9), there are insufficient data to support their recommendation. Therefore, we aimed to investigate the clinical course, including the management and outcomes, in patients with discordant results for genotypic and phenotypic DSTs for INH and RIF in a real-world practice.
Methods
Patients and data collection
In a single tertiary university hospital that manages about 400 TB patients annually, adult patients aged >18 years who had been diagnosed with the disease and who had a positive culture and available DST results for INH and RIF from August 2010 to October 2016 were screened. After reviewing their medical records, patients with discrepant genotypic-phenotypic DST results for either drugs were enrolled in this study. The institutional review board of the College of Medicine of the Catholic University of Korea approved this study (KC18RESU0758) and the need for informed consent was waived.
Patient data including the resistance pattern and clinical outcomes were collected. We focused on whether and how the drug regimen for TB management was changed by a clinician when a discrepant DST result was reported, and we evaluated the sequential treatment outcomes. The outcome was categorized following the 2103 WHO definitions (10). A favorable outcome included a cure or completion of the treatment for TB. A cure was defined as culture-negative status in the last month of treatment and on at least one prior test. Completion was defined as completing treatment without satisfying the criteria for a cure but with no evidence of failure. Relapse were defined as a diagnosis of TB disease during the 1-year follow-up period after a favorable outcome.
Genotypic and phenotypic DSTs
Specimens included respiratory and other tissue samples collected before anti-TB treatment. In all patients, the DSTs included both growth-based phenotypic assays and molecular-based genotypic assays using the initial collected samples. For the phenotypic DST, solid and liquid media such as Ogawa-Kudoh (Korean Institute of Tuberculosis, Osong, South Korea) and BACTEC 960 Mycobacterial Growth Indicator Tube (MGIT; Becton Dickinson, Sparks, MD, USA) were used to culture M. tuberculosis. The first isolated culture sample was sent to the Korean Institute of Tuberculosis, the national TB reference laboratory, and the phenotypic DST was performed using the absolute concentration method with Löwenstein-Jensen media. At the critical concentrations of 0.2 µg/mL for INH and 40 µg/mL for RIF, drug resistance to the corresponding drug was defined as M. tuberculosis growth >1% compared with the control (11). Drug susceptibility tests to other TB drugs were followed to a laboratory guideline for TB test in Korea (11).
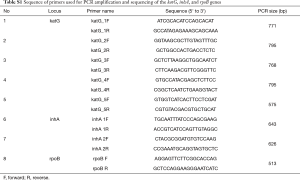
As for genotypic DST, the MTBDRplus assay was conducted for all patients. The test was performed using a sample cultured for M. tuberculosis. In samples with a positive acid-fast bacilli (AFB) smear with available bacterial burden, the test was performed using the smear sample to obtain a fast result. When there was a RIF discrepancy between the MTBDRplus test and the phenotypic DST, we also verified the result using the Xpert MTB/RIF test in some cases in which the two genotypic DSTs were applied. For repeated tests in a single patient, we included the results if they were performed at least 6 months apart or if the DST results were genotypic-phenotypic discrepant. The molecular assays were conducted following the manufacturers’ instructions. One case (subject 31) was carried out using DNA sequencing analysis for the two above drugs to identify a cause of discrepancy. It was performed by PCR method of various segments of 3 gene loci, known mutations conferring resistance to INH and RIF, katG codon 315 region and inhA promoter in the former and rifampicin resistance determining region or RRDR codons 508-534 of rpoB in the latter. Newly designed forward and reverse primers were used for PCR amplification (Table S1). Direct sequencing to amplified PCR product was performed in an ABI PRISM 3730XL Analyzer and results were evaluated by VariantReporter software v1.1 (Applied Biosystems, Foster City, CA, USA).
Full table
Statistical analysis
Data are expressed as n (%) using simple descriptive statistics. Using the phenotypic DST as the standard, the sensitivity, specificity, and positive and negative predictive values of the MTBDRplus test for INH or RIF resistance were calculated (12).
Results
Baseline characteristics of the study population
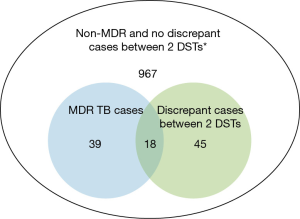
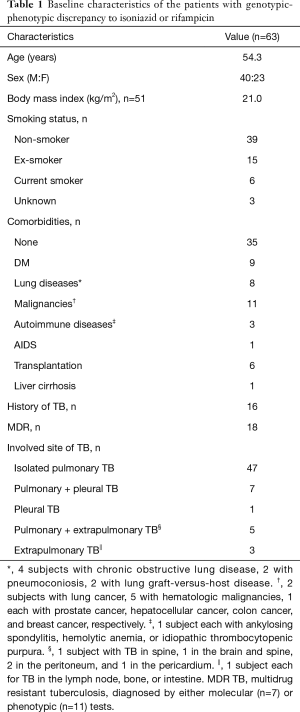
Of 1,069 TB patients whose results for genotypic and phenotypic DSTs for anti-TB drugs were verified, 63 (5.9%) had a discrepancy between the results of the 2 DSTs. In this cohort, 57 patients were diagnosed with MDR TB by one of the DSTs and 18 patients out of them (31.6%) showed discordant results for INH or RIF (Figure 1). The baseline characteristics of the 63 included patients are shown in Table 1. Their mean age was 54.3 years, and 40 (63.5%) were men. Sixteen patients had a history of TB and 12 had been taking anti-TB medication for at least 1 month at the time of use of the 2 DSTs. Eighteen of the 63 patients with discordant DST results (28.6%) were classified as having MDR TB based on the results from either DST (7 cases with a diagnosis by the MTBDRplus assay). Twenty-eight patients (44.4%) had resistance to at least 1 other anti-TB drug concurrently, as shown by the phenotypic DST.
Full table
Analysis of drug susceptibility results between the genotypic and phenotypic methods
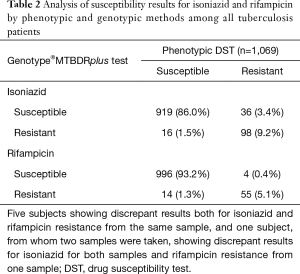
In the entire population of 1,069 patients, compared with the phenotypic DST, the sensitivity, specificity, and positive and negative predictive values of the molecular assay for INH were 74.2%, 98.3%, 86.0%, and 96.2%, respectively. The respective values for RIF were 93.2%, 98.6%, 79.7%, and 99.6%. Seventy samples from 63 patients had discordant results between the genotypic MTBDRplus test and the phenotypic DST; 52 showed a discrepancy for INH and 18 for RIF (Table 2). Five subjects showed discrepant results between the 2 DSTs for both INH and RIF. In one patient (number 31), 2 samples were discrepant; one of these had opposite susceptible results for both drugs, INH and RIF, and the other had a discrepancy for INH resistance between the genotypic and phenotypic tests. The most common pattern of disagreement was susceptibility in the genotypic DST, but resistance in the phenotypic DST for INH (n=36). The lowest frequency of disagreement was molecular susceptibility with phenotypic resistance for RIF (n=4). The Xpert MTB/RIF test was performed on 7 of the RIF-discrepant patients, whose results agreed with those from the MTBDRplus test (n=4) and with those from the phenotypic test (n=2). One was negative for TB PCR in the Xpert MTB/RIF.
Full table
Patient 31 is an interesting case. This patient was a 54-year-old man who had undergone bone marrow transplantation 2 months previously and was admitted with right hip pain. He had been prescribed INH prophylaxis because of a positive result in an interferon-gamma release assay 2 month earlier. The patient was diagnosed with bone TB and was treated with standard first-line drugs. The MTBDRplus test in cultured specimens for M. tuberculosis revealed no resistance to INH or RIF. Three months later, he complained of gait disturbance and was diagnosed with a brain abscess. Tissue biopsy showed positive AFB staining, and a phenotypic DST of the previous sample showed INH and ethambutol resistance. Assuming that his status represented a clinical failure to treat TB, the regimen was changed completely into new TB drugs, similar to those used for MDR TB treatment. At this time, the MTBDRplus test also showed no resistance to INH or RIF. Five months later, a phenotypic DST revealed resistance to both INH and RIF, which confirmed MDR TB. To investigate the cause of the discrepant results, we performed DNA sequencing analysis for INH and RIF mutations. The analysis identified 2 mutations, S315N for katG gene and L490P for rpoB gene, which cannot be detected in the MTBDRplus test (Figure S1).

Clinical profiles and treatment outcome in the 63 TB patients with discrepant results for INH or RIF
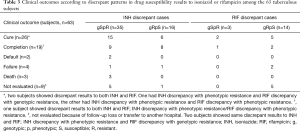
Table 3 shows clinical outcome of the total 63 patients with INH or RIF discrepancy. Fifty-four patients were managed for TB in our hospital, and 45 (83.3%) had a favorable outcome such as being cured (n=26) or completing the treatment (n=19). The mean treatment durations were 14.0 and 10.9 months in patients with a favorable outcome with and without MDR TB, respectively. None of the 40 patients who could be followed up for 1 year after the treatment showed evidence of TB recurrence.
Full table
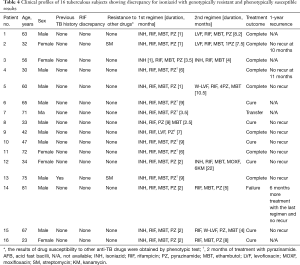
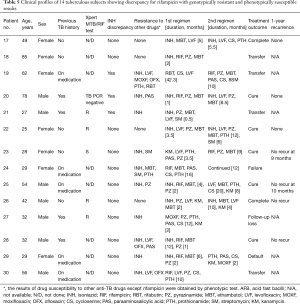
We focused on those with genotypically resistant and phenotypically susceptible patterns for INH or RIF. The clinical data of each group are shown in Tables 4,5, respectively. In the discrepant group with genotypic resistance to INH, all patients except 1 did not have any prior TB history. None of the samples from these patients exhibited discrepant results for RIF, although 2 patients had concurrent resistance to streptomycin. Ten of 16 patients continued taking the usual dose (300 mg/day) of INH for more than the standard period of 6 months, and their treatment outcomes were mostly favorable and without recurrence. By contrast, in the discrepant group with genotypic resistance to RIF, 9 of 14 patients had a past TB history (n=4) or were taking anti-TB medication (n=5) at the time of the DSTs. Half of them (7/14) were diagnosed MDR TB by the genotypic MTBDRplus test. Ten patients also had INH resistance as shown by one of the DSTs, and 3 of these patients exhibited discrepancy for INH between the 2 DSTs. In 8 of 14 patients, the clinicians changed RIF to other drugs after noticing the genotypic resistance to RIF. In addition, 5 out of 7 patients with a favorable outcome discontinued RIF. Among 7 patients (numbers 23–25 and numbers 27–30) with genotypically confirmed MDR TB, 3 (number 23, 25, and 28) were cured; 2 of these 3 had been treated with RIF.
Full table
Full table
Discussion
In the current study, we found a low overall frequency (5.9%) for discrepant results of DSTs for INH or RIF between the genotypic MTBDRplus test and the phenotypic test in South Korea, a country with intermediate TB incidence. However, in the 57 patients with an MDR TB diagnosis, there was a high percentage of discordance (31.6%, n=18). The most frequently encountered discordant pattern was genotypic susceptibility and phenotypic resistance to INH. The clinical outcomes of the treated patients were mostly favorable, and the mean treatment durations were 14 and 10 months with or without including MDR TB cases, respectively.
Genotypic DSTs for anti-TB drugs based on the molecular method have the clinical advantages of rapid return of results and safety during the procedure. However, the discrepancy with the phenotypic DST in a real practice poses a clinical dilemma. Even though the incidence is low, as in our study, the potential clinical impact cannot be ignored. If the susceptibility shown by the genotypic DST is a false positive (false resistant), the patient would be prescribed an inappropriate drug for an unnecessarily long time, which increases the risk of side effects and lowers the efficacy. Similarly, if the susceptibility shown by the genotypic DST is a false negative (false susceptible), the patient would also be prescribed an incorrect drug, which would be ineffective and could induce resistance to that drug and, consequently, treatment failure. In particular, the former situation involving a false-positive result by genotypic DST is considered to be more confusing and challenging in choosing proper regimens for TB, to both the patient and clinician.
In this study, physicians managed most of the patients with genotypically resistant and phenotypically susceptible results to INH by maintaining the drug for an extended duration, and the patients had a good clinical outcome. This finding differs from that of Jo et al. (13), who exchanged INH for later-generation fluoroquinolones in a similar group of patients and found favorable results. Intriguingly, we observed that, in the RIF-discrepant group with genotypical resistance, many patients had peculiar features such as exposure to anti-TB medication and an MDR TB diagnosis. Clinicians usually treated these patients by replacing RIF with other drugs including an MDR regimen with a longer duration. Other reports have also shown that, in the case of RIF discordance with genotypic resistance, treatment failure develops more frequently, and an extended-duration or second-line anti-TB regimen might be more successful (14-16).
Various explanations have been suggested for the genotypic–phenotypic discrepancy in INH or RIF susceptibility. These include rare gene mutations such as those in kasA or mshA for INH susceptibility, mutations at other regions of katG and inhA or rpoB outside regions sequenced by the MTBDRplus assay, silent mutations, technical errors, disputed mutations leading to increased minimal inhibitory concentration below the critical concentration in some phenotypic DSTs, inadequate phenotypic results, heteroresistance, and random errors (8,13,17). In our study, patient 31 with bone and brain TB showed genotypic susceptibility with phenotypic resistance to INH and RIF. DNA sequencing analysis detected new mutations of Ser315Asn (S315N) in katG and Leu490Pro (L490P) for rpoB, both of which are not covered by the commercial line probe assay.
Recent studies have noted that some noncanonical rpoB mutations confer low-level resistance or are associated with susceptibility when tested using the phenotype methods, especially those using liquid medium, but that these mutations are also associated with adverse treatment outcomes (18,19). Mathys et al. (20) reported that a silent mutation in rpoB resulted in false-positive RIF resistance in the Xpert MTB/RIF assay. Another patient in our study (number 32) had phenotypically both resistant and susceptible results in 2 samples, obtained 1 month apart, with genotypic susceptibility to INH, which suggested inconsistency in the phenotypic DST. The older culture-based phenotypic assay has some problems such as strict prerequisites for reproducible results and using a critical concentration that is not based on pharmacokinetic/pharmacodynamic data (17,21,22). Our study included another interesting case (patient 23) involving resistance to RIF shown in the MTBDRplus assay but susceptibility shown in the phenotypic DST. The Xpert MTB/RIF test showed susceptibility to RIF, which indicated discordance between the 2 molecular methods. Rahman et al. (23) reported that the Xpert MTB/RIF assay is more accurate than the MTBDRplus assay for detecting RIF susceptibility in discrepant cases. By contrast, Rufai et al. (24) showed that a rare mutation, L533P in rpoB, could be missed by the Xpert test and may be detected only by the MTBDRplus test. Other researchers have suggested that DNA sequencing analysis may be helpful for identifying the reasons for discrepant DST results between tests (8,13,17,22,25). However, in a real practice, it is difficult to differentiate the reasons leading to discrepant DST results among the causes mentioned above and to analyze all discordant samples. Therefore, a good alternative may be to repeat the phenotypic or genotypic test with clinically thorough consideration of TB course (4).
In the current study, the most common pattern of discrepancy was genotypic susceptibility and phenotypic resistance to INH, which is a similar to findings reported from the USA (14,26). This phenomenon is reasonable because whereas MTBDRplus test can detect specific mutations at katG and inhA genes, 20% of INH resistance is known to be developed by mutations at regions other the above two genes, such as ahpC gene (27). Intriguingly, RIF-discordant cases with genotypic resistance also showed INH resistance in either the MTBDRplus assay or the phenotypic test. This result is consistent with that reported by Shah et al. (14), who found that 14 of 16 patients (88%) with rpoB mutations had INH resistance on the phenotypic DST. This finding may mean that, despite discordant cases, genotypic resistance to RIF may be more serious than resistance to INH, as in concordant cases. In addition, it is notable that the patients with MDR TB in our study were 5 times more likely to have a discrepant DST compared with all TB patients. Heteroresistance is one possible mechanism to explain a high rate of discrepancy in MDR TB patients because it occurs more frequently in high-prevalence regions for MDR TB and in previously treated patients (8).
Our study has some weaknesses. First, it was an observational and retrospective study with a small group of patients, so we cannot conclude clearly through statistical analyses which test results for DST to anti-TB drugs would be more strongly correlated with clinical outcomes in patients with a genotype-phenotype discrepancy for INH or RIF. Second, we did not perform DNA sequencing of gene mutations related to INH or RIF resistance to confirm the resistance and to identify specific mutations and causes of discordance. Recent reports have shown that strains with low-level resistance for RIF, which shows genotypical resistant but phenotypical susceptible, have a portion of 10–22% of treatment failure cases, suggesting that the current recommended critical concentration to RIF should be reviewed (18,28). On the other hand, Kambli et al. showed that phenotypic DST via quantitative MICs had good correlations with specific genetic mutations to INH or RIF (29). In resource-limited settings where DNA sequencing could not be performed, combining the analysis with the results of the phenotype test via the quantified minimum inhibitory concentration may increase the accuracy of the relationships. Finally, in 7 patients, MTBDRplus assay used on smear samples, not sample cultured for M. tuberculosis to allow for the rapid acquisition of DST results and this may have affected the final results in the current study. We reviewed discrepant results obtained using MTBDRplus method in these patients and compared them with those in the total group. Out of 8 discordant results to INH or RIF, 4 indicated genotypic susceptibility with phenotypic resistance to INH and 4 indicated genotypic resistance with phenotypic susceptibility to RIF. The former pattern of discrepancy was also the most common type in all patients, and it seems unlikely that the use of smear samples in the MTBDRplus assay affected the final results. However, in the latter cases of RIF discrepancy with genotypic resistance, considerable proportion (4/14 cases) were detected using the MTBDRplus method with smear samples compared with the total number (14) of the same pattern of discrepancy. We reviewed the results of the Xpert MTB/RIF performed at a similar time in these 4 patients and found that 3 patients showed concordant data for RIF resistance with the MTBDRplus results. Therefore, we conclude that there was a small chance that MTBDRplus tests using smear samples may have influenced the final discrepant results of DSTs.
Conclusions
Genotypic–phenotypic discrepancy for INH or RIF is not common in South Korea, a country with intermediate TB incidence. However, the rate of discrepancy was 5 times higher in patients with MDR TB. Considering the clinical situation of TB patients, RIF discordance with genotypic resistance may have a greater impact on treatment outcome than INH discordance. At present, there are insufficient data to determine how and to what extent the discordance would affect TB management. Therefore, if a discrepancy occurs, clinicians should act cautiously and comprehensively by considering both the DST results and clinical factors, and by repeating the test or applying further DNA sequencing.
Acknowledgements
Funding: This work was supported by the Catholic Medical Center Research Foundation in the program year of 2015 and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI14C1234).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of the College of Medicine of the Catholic University of Korea approved this study (KC18RESU0758) and the need for informed consent was waived.
References
- World Health Organization. Global tuberculosis report 2018. Geneva: World Helath Organization; 2018.
- Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea 2016. Cheongju, Korea: Korea Centers for Disease Control and Prevention; 2016.
- Joh JS, Lee CH, Lee JE, et al. The Interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci 2007;22:26-9. [Crossref] [PubMed]
- Domínguez J, Boettger EC, Cirillo D, et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis 2016;20:24-42. [Crossref] [PubMed]
- Ling DI, Zwerling AA, Pai M. GenoType MTBDRassays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J 2008;32:1165-74. [Crossref] [PubMed]
- Noor KM, Shephard L, Bastian I. Molecular diagnostics for tuberculosis. Pathology 2015;47:250-6. [Crossref] [PubMed]
- Ahmad S, Mokaddas E. Current status and future trends in the diagnosis and treatment of drug-susceptible and multidrug-resistant tuberculosis. J Infect Public Health 2014;7:75-91. [Crossref] [PubMed]
- Hofmann-Thiel S, Hoffmann H, Hillemann D, et al. How should discordance between molecular and growth-based assays for rifampicin resistance be investigated? Int J Tuberc Lung Dis 2017;21:721-6. [Crossref] [PubMed]
- Cabibbe AM, Sotgiu G, Izco S, et al. Genotypic and phenotypic M. tuberculosis resistance: guiding clinicians to prescribe the correct regimens. Eur Respir J 2017;50:1702292. [Crossref] [PubMed]
- World Health Organization (WHO). Definitions and Reporting Framework for Tuberculosis. (revision) 2013. Available online: http://apps.who.int/iris/bitstream/handle/10665/79199/9789?sequence=1
- Korea Centers for Disease Control and Prevention, Korea National Institute of Health, The Korean Society of Clinical Microbiology. Manual of Laboratory Tests for Tuberculosis. 2013.
- Gordis L. Epidemiology. 5th ed. Philadelphia: Saunders; 2013.
- Jo KW, Yeo Y, Sung H, et al. Analysis of discrepant results between the Genotype®MTBDRplus assay and an antimicrobial drug susceptibility test for isoniazid-resistant tuberculosis. Respir Med 2017;122:12-7. [Crossref] [PubMed]
- Shah NS, Grace Lin SY, Barry PM, et al. Clinical Impact on Tuberculosis Treatment Outcomes of Discordance Between Molecular and Growth-Based Assays for Rifampin Resistance, California 2003-2013. Open Forum Infect Dis 2016;3:ofw150. [Crossref] [PubMed]
- Pang Y, Ruan YZ, Zhao J, et al. Diagnostic dilemma: treatment outcomes of tuberculosis patients with inconsistent rifampicin susceptibility. Int J Tuberc Lung Dis 2014;18:357-62. [Crossref] [PubMed]
- Williamson DA, Roberts SA, Bower JE, et al. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2012;16:216-20. [Crossref] [PubMed]
- Schön T, Miotto P, Köser CU, et al. Mycobacterium tuberculosis drug-resistance testing: challenges, recent developments and perspectives. Clin Microbiol Infect 2017;23:154-60. [Crossref] [PubMed]
- Van Deun A, Aung KJ, Bola V, et al. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 2013;51:2633-40. [Crossref] [PubMed]
- Rigouts L, Gumusboga M, de Rijk WB, et al. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol 2013;51:2641-5. [Crossref] [PubMed]
- Mathys V, van de Vyvere M, de Droogh E, et al. False-positive rifampicin resistance on Xpert® MTB/RIF caused by a silent mutation in the rpoB gene. Int J Tuberc Lung Dis 2014;18:1255-7. [Crossref] [PubMed]
- Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J 2005;25:564-9. [Crossref] [PubMed]
- Ocheretina O, Escuyer VE, Mabou MM, et al. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One 2014;9:e90569. [Crossref] [PubMed]
- Rahman A, Sahrin M, Afrin S, et al. Comparison of Xpert MTB/RIF Assay and GenoType MTBDRplus DNA Probes for Detection of Mutations Associated with Rifampicin Resistance in Mycobacterium tuberculosis. PLoS One 2016;11:e0152694. [Crossref] [PubMed]
- Rufai SB, Kumar P, Singh A, et al. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol 2014;52:1846-52. [Crossref] [PubMed]
- Mokaddas E, Ahmad S, Eldeen HS, et al. Discordance between Xpert MTB/RIF assay and Bactec MGIT 960 Culture System for detection of rifampin-resistant Mycobacterium tuberculosis isolates in a country with a low tuberculosis (TB) incidence. J Clin Microbiol 2015;53:1351-4. [Crossref] [PubMed]
- Yakrus MA, Driscoll J, Lentz AJ, et al. Concordance between molecular and phenotypic testing of Mycobacterium tuberculosis complex isolates for resistance to rifampin and isoniazid in the United States. J Clin Microbiol 2014;52:1932-7. [Crossref] [PubMed]
- Jagielski T, Bakula Z, Roeske K, et al. Mutation profiling for detection of isoniazid resistance in Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother 2015;70:3214-21. [PubMed]
- Yip CW, Leung KL, Wong D, et al. Denaturing HPLC for high throughput screening of rifampicin- resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2006;10:625-30. [PubMed]
- Kambli P, Ajbani K, Sadani M, et al. Defining multidrug-resistant tuberculosis: correlating GenoType MTBDRplus assay results with minimum inhibitory concentrations. Diagn Microbiol Infect Dis 2015;82:49-53. [Crossref] [PubMed]


