Completely thoracoscopic surgical stabilization of rib fractures: can it be done and is it worth it?
The practice of surgical stabilization of rib fractures (SSRF) began as a rare, last ditch operation for patients with the most severe chest wall disruptions (1). However, over the last two decades, the surgery has evolved into one that is performed routinely, early in the post injury course, and with the intention of mitigating primarily long-term outcomes such as pain, return to work, and recreational activities (2). With this change in scope has come a change in the technique of the surgery, which has, in many ways, mirrored the changes observed in elective thoracic surgery. Specifically, and in recognition of the deleterious effects of additional tissue trauma, larger thoracotomy incisions have given way to minimally invasive, muscle sparring ones. In addition, a transition has occurred with respect to both position and direction of incisions to expose the chest wall. Whereas the primarily transverse posterolateral thoracotomy affords excellent exposure to intra-thoracic structures, it provides relatively limited exposure to a series of more than four rib fractures. By contrast, primarily vertical incisions, centered over muscle margins, allow adequate exposure to repair up to seven ribs without extensive dissection. Advancements in equipment used to perform SSRF, including right angled drill and screw systems, have occurred in parallel, and further allowed for minimization of tissue trauma.
The logical extension of the progression toward minimally invasive SSRF is thoracoscopic reduction and fixation. Completely thoracoscopic SSRF has only been performed in a handful of cases, including by the author (3), and with difficulty, mostly related to both inexperience with the technique and limitations of equipment. Although both of these obstacles will eventually be surpassed, it remains to be seen if completely thoracoscopic SSRF confers any outcome benefit to patients as compared to contemporary, minimally-invasive, extra-thoracic SSRF. The theoretical advantages of completely thoracoscopic SSRF are summarized in Table 1.

Full table
To what does completely thoracoscopic SSRF refer?
Video-assisted thoracoscopic surgery (VATS) is now routinely practiced in both trauma and thoracic surgery. Theoretical advantages of employing VATS routinely during SSRF, beyond repair of the rib fractures, are discussed below. However, for the purposes of this article, completely thoracoscopic SSRF refers to intra-thoracic reduction, drilling, and plate placement to the inner cortex of the rib under thoracoscopic visualization. This distinction is relevant as several innovative techniques have been recently described that employ variations of both camera visualization and fracture and plate manipulation but do not, using the aforementioned definition, constitute completely thoracoscopic SSRF. For example, Merchant and Onugha described a technique of elevating sub-muscular, extra-thoracic flaps using a balloon dilator, followed by insufflation of this space and camera visualization and repair of rib fractures (remaining extra-thoracic) (4,5) (Figure 1). Furthermore, an intra-thoracic plate-based system was recently approved for use by the Food and Drug Administration that involves combined intra and extra thoracic passage of wires to secure permanent plates to the inner cortex of ribs using grooves (6). Such innovations represent important steps in the progression of SSRF that are promising and will require outcomes validation. However, for the purposes of this discussion, they are not considered completely thoracoscopic SSRF.
Advantages of completely thoracoscopic SSRF related to rib fracture repair
Advantages to completely thoracoscopic reduction and fixation of rib fractures, as compared to both extra-thoracic and combined approaches, may be grouped broadly into improved exposure of rib fractures, minimization of trauma/injury to extra-thoracic structures, and minimization of trauma/injury to intra-thoracic structures.
Thoracoscopy affords wide visualization of the entire chest wall. This improved exposure is particularly useful when addressing structures that are normally both difficult and morbid to access using open techniques. One example of this concept in elective thoracic surgery is VATS intrathoracic first rib resection for thoracic outlet syndrome (7). These same advantages may be realized when repairing rib fractures, and, in particular, in two of the more challenging areas to adequately expose: subscapular fractures and very posterior fractures.
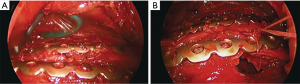
In the case of the former, although patient positioning may aid in either medialization or lateralization of the scapula to provide access to fractures under the lateral and medial body, respectively (8), some degree of scapula retraction is usually necessary to adequately expose and repair sub-scapular fractures. Scapular retraction typically involves both division of muscular attachments and a fair amount of force on the bone itself, which, in up to 20% of cases, is also fractured (9). Moreover, despite optimal positioning and retraction, extra-thoracic repair of subscapular rib fractures located underneath the middle of the scapular body remains challenging. By contrast, the inner cortex of the sub-scapular ribs is readily visible and accessible via thoracoscopy (Figure 2). Although a thoracoscopic approach to reduction and repair of these fractures remains limited by the presence of the scapula (discussed further in the techniques section), the task of adequate exposure is greatly simplified.
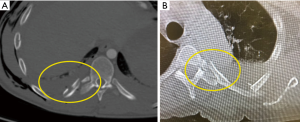
Posteriorly, the tubercle of the rib articulates with the transverse costal facet of the vertebrae, and the head of the rib articulates with the superior costal facet of the vertebrae. The neck of the rib is a 3–5 cm segment between the head and tubercle that is covered externally by the transverse process of the vertebrae. Accordingly, external exposure of the neck of the rib is almost never performed, due to the need to disarticulate it from the transverse process. Because of this limitation, very posterior fractures (Figure 3A) are usually managed non-operatively, regardless of degree of displacement, or even proximity to major structures such as the aorta (Figure 3B). Such exposure would be required not only for fractures of the neck itself (which are relatively rare), but also for fractures of the tubercle, angle, and proximal body of the rib, which, at a minimum require 3 cm of proximal exposure to appropriately land a plate. Although the clinical utility of repairing very posterior rib fractures remains unknown, their exposure is unquestionably improved via a thoracoscopic approach to the inner cortex.
The ribs are covered by the broad-based muscles of the chest wall, including the pectoralis major and minor, latissimus dorsi, serratus anterior and posterior, trapezius, and rhomboids. Postero-superiorly, the scapula and its muscles cover the ribs, and postero-medially, the erector spinae muscles cover the tubercle, angles, and proximal rib bodies. Much progress had been made in terms of developing muscle sparring approaches to SSRF (8,10,11). In general, anterior fractures may be approached in the supine position by developing a sub-pectoral flap. Lateral fractures may be approached in the lateral decubitus position by developing a sub-latissimal flap (beginning at the medial border of the muscle), and then splitting the serratus anterior muscle bellies. Finally, posterior fractures may be approached in the prone position and through the triangle of auscultation by developing both sub-trapezial and sub-latissimal flaps. Although less morbid than earlier iterations that utilized muscle division, these exposures are still limited by several drawbacks. First, the development of large, sub-muscular flaps likely increases the risk of both seroma formation and infection. Next, access to a series of fractures through a single exposure typically tops off at six. Moreover, the posterior approach described is ideal for fractures of ribs 5–8; fractures of posterior ribs three and four almost always requires at least partial division of the rhomboid, trapezius, and erector musculature. Next, in the case of more than one fracture series, multiple incisions/exposures are usually necessary. For example, a flail chest involving both antero-lateral and postero-lateral fracture series would need to be approached either via two separate incisions (e.g., anterior/supine and posterior/prone) or a traditional postero-lateral thoracotomy with muscle division.
In addition to the musculature of the chest wall, several nerves are at risk during traditional, extra-thoracic exposure. The nerves most commonly encountered during SSRF are the long thoracic nerve during high lateral and axillary exposures, spinal accessory nerve during high posterior exposures, and the lateral cutaneous branches of the intercostal nerves in both. Although these nerves course in a usual location, reports of injury leading to scapular and abdominal muscular dysfunction have been reported (12,13). A thoracoscopic approach to the inner cortices of the ribs mitigates each of these concerns.
Intra-thoracic structures at risk during SSRF include the lung and heart. Most current fixation platforms employ bicortical fixation, meaning that the screws are required, at a minimum, to barely “stick out” of the opposite cortex from which the direction of fixation occurs. Intra-operative maneuvers, such as measuring the depth of the rib, use of drill guides, and single lung ventilation, help to mitigate the risk of either drilling or screwing too far beyond the inner cortex of the rib and injuring either the lung or heart, but this risk remains and, in the course of the later, may be catastrophic. Direct visualization of the inner cortex of the rib, in the setting of contra-lateral lung ventilation employed during thoracoscopy, should minimize the risk of either cardiac or pulmonary injury during SSRF.
The incidence of serious hardware complications following SSRF is beginning to be quantified and, fortunately, appears to be relatively rare (14). Partial or complete hardware dislodgement represents one such complication that has been encountered by the author. In this case, a patient presented as an outpatient to clinic with an uncomfortable bulge over his right pectoralis muscle six weeks following SSRF of an anterior fracture series. Physical exam demonstrated a palpable, subcutaneous plate, and removal was accomplished in the operating room without incident (the underlying fracture had since healed) (Figure 4). Given the increased utilization of SSRF as a treatment modality for rib fractures, the prevalence of hardware dislodgements will likely increase. Furthermore, patients with a relative paucity of muscle and fat may be able to palpate their plates, even when positioned properly. Some patients may experience distress in this situation. Finally, extra-thoracic plate placement in the sub-scapular position may cause additional morbidity as a result of the plates grinding along the body of the scapula during shoulder movement, particularly in the case of a coexisting scapular body fracture. Placement of hardware on the inner cortex of the rib would theoretically eliminate the morbidity associated with these three scenarios. Using the congenital chest wall deformity literature as an example, there does not appear to be an adverse consequence of intra-pleural hardware placement long term, including when dislodged (15).
Advantages of thoracoscopy during SSRF, exclusive of rib fracture repair
Regardless of the technique used to repair rib fractures (i.e., extra-thoracic, intra-thoracic, or combined), there are several potential advantages to the routine use of thoracoscopy during SSRF. The first involves fracture identification. Multiple methods for intra-operative localization of rib fractures have been reported, including fluoroscopy, ultrasonography, surface anatomy, and pre-operative imaging, including 3-dimensional (3D) reconstruction of CT scans; each method has both advantages and disadvantages (16). One disadvantage of relying only on pre-operative imaging is that fractures may incur interval displacement. In this case, real time, intra-operative assessment of all fractures is advantageous. In practice, incisions are usually planned around the muscles of the chest wall, as opposed to the fractures themselves (i.e., incisions are usually not made directly over each fracture). However, a comprehensive overview of the inner chest wall, including all fracture locations, may aid in refining the planned incision; for example, moving either superiorly or inferiorly along the medial border of the latissimus dorsi muscle. This technique may be particularly useful in obese patients.
Retained hemothorax is present in approximately one half of patients at the time of SSRF (9). In general, early evacuation of moderate to large hemothorax in blunt trauma patients via VATS both improves outcomes and is cost effective, as compared to either observation or bedside tube thoracostomy (17,18). Furthermore, evacuation of retained hemothorax, including pleural irrigation with sterile saline, at the time of SSRF, is associated with a decreased post-operative incidence of both retained hemothorax and empyema (19). Thoracoscopic evacuation of hemothorax at the time of SSRF adds relatively little time to the operation and can be accomplished with the satisfaction of direct visualization of all of the pleural surfaces.
Routine thoracoscopy also affords the opportunity to explore the thoracic cavity for additional injuries. Lung, diaphragmatic, and intercostal arterial injuries all represent examples of such injuries upon which intervention may occur at the time of surgery. There is some preliminary evidence that resection/repair of lung parenchymal injury at the time of VATS for evacuation of retained hemothorax decreases both duration of post-operative chest tubes and infection risk (20).
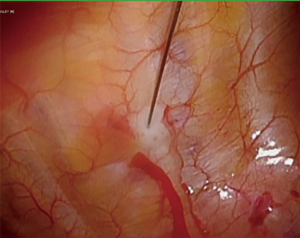
Early and adequate loco-regional anesthesia is imperative to the successful management of patients with severe chest wall injuries. Multiple modalities exist, ranging from thoracic epidural catheters to intercostal nerve blocks. In the author’s experience, both thoracic and para-vertebral catheters are subject to both myriad contra-indications (e.g., coagulopathy and spine fractures) and side effects (e.g., sympatholysis). For these reasons, intra-operative inter-costal nerve block with liposomal bupivacaine is performed at the time of SSRF. Using a 7-inch, 22-gauge needle, the sub-pleural space is infiltrated with anesthesia, which lasts for 2–4 days post-operatively (Figure 5). Indwelling catheters may also be placed in the extra-pleural plane for purposes of post-operative instillation of local anesthesia. Direct visualization of the proper plane of injection is likely more effective than the relatively “blind” technique employed in placing percutaneous catheters at the bedside (21). Finally, chest tubes may be placed under direct, thoracoscopic visualization, minimizing inadvertent placement in undesirable locations, such as the pulmonary fissures and costo-diaphragmatic recesses.
One final advantage of thoracoscopy during SSRF involves education of surgical trainees. As trauma continues to evolve into a primarily non-operative discipline, the challenge of technical skill acquisition for medical students, residents, and fellows continues to grow. This dilemma is particularly relevant in the case of advanced laparoscopy/thoracoscopy, which is now contained within the American Association for the Surgery of Trauma’s Acute Care Surgery Curriculum (22). Routine thoracoscopy during SSRF allows these learners additional exposure to thoracoscopy when the stakes are not as high as, for example, either VATS lobectomy or esophagectomy. Finally, projection of thoracoscopy on to multiple monitors allows for easy viewing for trainees in the room who may not be participating directly in the surgery. Both photographs and videos of the surgeries may also be used for teaching and research purposes.
Technical considerations
Technical considerations in accomplishing completely thoracoscopic SSRF are drawn from the author’s experience with this operation (3), as well as elective thoracic surgery. Patient selection is relevant in so much as the surgeon’s first cases should be of relative technical ease (i.e., lateral fractures of ribs 6–8).
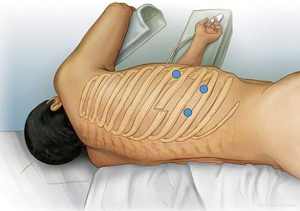
The surgery is performed under general anesthesia and requires lung isolation, which may be accomplished by any of the standard modalities. Because neither hilar nor lung parenchymal dissection is required, the author has found that a bronchial blocker in conjunction with low pressure pleural space insufflation is almost always adequate. The lateral decubitus position likely provides the most comprehensive exposure to the inner chest wall, and is preferred in all cases, with the exception of isolated very anterior or posterior fracture series, in which case supine and prone, respectively, may be considered. The table is flexed at the patient’s ipsilateral anterior superior iliac spine to widen the rib spaces and allow for maximal camera maneuverability. A minimum of three incisions will be required: one for the camera, one to position and hold the plate, and one to operate both the drill and screwdriver. Incisions should be placed as to be able to triangulate instrumentation and with the eventual passage of the chest tube in mind (Figure 6).
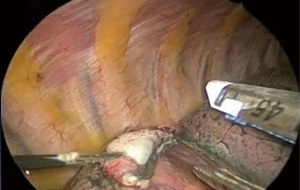
The author prefers to use laparoscopic trocars and attach insufflation tubing to achieve low pressure (e.g., 6–10 mmHg) pleural space insufflation. This maneuver aids in both lung desufflation and mediastinal shifting away from the field. Prior to addressing the rib fractures, a thorough exploration of the pleural cavity is undertaking in order to identify any additional pathology. Then, the fractures are noted and exposed by opening up the underlying pleura using cautery. This step need be done only to the extent that the precise location of the ribs is understood; excessive stripping of the periosteum is not recommended.
The next task is reduction, which, along with fixation, is currently the most challenging step of the operation. The author uses heavy suture, passed around both rib fracture fragments through a stab incision and using a laparoscopic suture passer, to accomplish this task. However, this maneuver will not be possible for sub-scapular fractures, for which internal reduction is required, and may be accomplished using two laparoscopic Kittner (peanut) blunt dissectors. This may require placement of a fourth port. With the rib fracture now in reduction, a plate is selected. All but one currently available platform utilizes plates that are both pre-contoured and intended to be placed on the outer cortex of the rib. As such, the plate will need to be bent in the reverse direction. Multiple tools are available to assist in this task, including using a second, pre-bent plate turned upside down to match the contour, and, if available, utilizing a sterilized 3D print of the contra-lateral rib. This latter step, in conjunction with the CT scan, may also be helpful in determining the thickness of the rib.
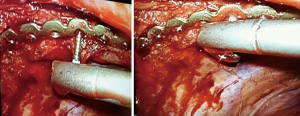
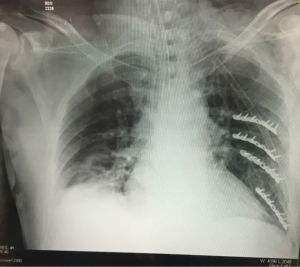
Once the plate is bent, it is introduced into the pleural space with a ratcheted grasper and the ties are loosed. The plate is placed between the rib fracture fragments and the ties, and the ties are cinched down, pinning the plate in position. Drilling and screw placement are then accomplished using a right-angle system (Figure 7). The pleural cavity is irrigated, a chest tube is placed, and the incisions are closed. Post-operative chest XRAY demonstrates the characteristic appearance of the plates with the screws pointing inwards (Figure 8).
The technology for completely thoracoscopic SSRF remains rudimentary, particularly in light of the advancements that have been made in robotic thoracic surgery. In particular, articulating drills and screwdrivers are necessary to allow for fixation at a right angle to the chest wall. Furthermore, screwdrivers with “quick release” functions will be useful in minimizing loss of screws within the thoracic cavity. Finally, development of rib-specific plates that are contoured to match the inner curve of the rib would eliminate the time necessary to bend the plates intra-operatively.
Outcomes of thoracoscopic SSRF
Although several theoretical advantages to completely thoracoscopic SSRF have been detailed, there are no current studies that have compared this technique directly to either extra-thoracic SSRF or non-operative management. Such studies will need to focus on the typical parameters comparing open to minimally invasive surgery, including operative time, learning curves, post-operative pain scores, length of stay, complications, and cost. Ultimately, several studies will be needed to address specific patient populations, fracture patterns, and surgeon expertise. Pending such data, thoracoscopic SSRF cannot be considered standard of care, and utilization of this technique should occur in the setting of a research protocol.
Despite these limitations, completely thoracoscopic SSRF is possible, and has been performed. Moving forward, close collaboration between surgeons and industry will be required to provide feedback in terms of the design of instrumentation to move the operation forward.
Summary
The penetrance of SSRF into many trauma and thoracic surgeons’ practice has led to an explosion of ingenuity with respect to operative technique. Surgeons with pre-existing, extensive experience with thoracoscopy are asking how to translate the benefits to SSRF. Unfortunately, the current landscape represents a case of the theoretical preceding the possible, and, in many ways, the technology needs to catch up to the surgeon’s ability. However, several projects are currently underway with a specific focus on thoracoscopic SSRF instrumentation. At a minimum, surgeons who perform SSRF should consider incorporating VATS into their operation routinely, for the benefit of both their and their trainees’ operative skills. However, the most important potential benefit belongs to the patient, and this benefit remains to be seen. The onus is upon the academic surgical community to catalogue their experience with thoracoscopic SSRF, as compared to both extra-thoracic and non-operative management, in order to conduct a meaningful analysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. FM Pieracci is a paid educator for DePuy Synthes and receives research funding from DePuy Synthes.
References
- Bemelman M, Poeze M, Blokhuis TJ, et al. Historic overview of treatment techniques for rib fractures and flail chest. Eur J Trauma Emerg Surg 2010;36:407-15. [Crossref] [PubMed]
- Kane ED, Jeremitsky E, Pieracci FM, et al. Quantifying and exploring the recent national increase in surgical stabilization of rib fractures. J Trauma Acute Care Surg 2017;83:1047-52. [Crossref] [PubMed]
- Pieracci FM, Johnson JL, Stoval RT, et al. Completely Thoracoscopic, Intra-pleural Reduction and Fixation of Severe Rib Fractures. Trauma Case Reports 2015;1:39-43. [Crossref] [PubMed]
- Merchant NN, Onugha O. Extra-Thoracic Video-Assisted Thoracoscopic Surgery Rib Plating and Intra-Thoracic VATS Decortication of Retained Hemothorax. Surg Technol Int 2018;33:251-4. [PubMed]
- Merchant NN, Onugha O. Novel extra-thoracic VATS minimally invasive technique for management of multiple rib fractures. J Vis Surg 2018;4:103. [Crossref] [PubMed]
- SIG Medical wins FDA nod for Advantage Rib fracture repair device. Available online: https://www.massdevice.com/sig-medical-wins-fda-nod-for-advantage-rib-fracture-repair-device/
- Hwang J, Min BJ, Jo WM, et al. Video-assisted thoracoscopic surgery for intrathoracic first rib resection in thoracic outlet syndrome. J Thorac Dis 2017;9:2022-8. [Crossref] [PubMed]
- Pieracci FM, Rodil M, Stovall RT, et al. Surgical stabilization of severe rib fractures. J Trauma Acute Care Surg 2015;78:883-7. [Crossref] [PubMed]
- Pieracci FM, Lin Y, Rodil M, et al. A prospective, controlled clinical evaluation of surgical stabilization of severe rib fractures. J Trauma Acute Care Surg 2016;80:187-94. [Crossref] [PubMed]
- Ali-Osman F, Mangram A, Sucher J, et al. Geriatric (G60) trauma patients with severe rib fractures: Is muscle sparing minimally invasive thoracotomy rib fixation safe and does it improve post-operative pulmonary function? Am J Surg 2018;216:46-51. [Crossref] [PubMed]
- Hasenboehler EA, Bernard AC, Bottiggi AJ, et al. Treatment of traumatic flail chest with muscular sparing open reduction and internal fixation: description of a surgical technique. J Trauma 2011;71:494-501. [Crossref] [PubMed]
- Skedros JG, Mears CS, Langston TD, et al. Medial scapular winging associated with rib fractures and plating corrected with pectoralis major transfer. Int J Surg Case Rep 2014;5:750-3. [Crossref] [PubMed]
- Ting M, Gonzalez J. Something Is Growing Inside: An Unusual Adverse Effect of Paravertebral Block. Reg Anesth Pain Med 2017;42:537-8. [Crossref] [PubMed]
- Thiels CA, Aho JM, Naik ND, et al. Infected hardware after surgical stabilization of rib fractures: Outcomes and management experience. J Trauma Acute Care Surg 2016;80:819-23. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Pieracci FM, Majercik S, Ali-Osman F, et al. Consensus statement: Surgical stabilization of rib fractures rib fracture colloquium clinical practice guidelines. Injury 2017;48:307-21. [Crossref] [PubMed]
- Smith JW, Franklin GA, Harbrecht BG, et al. Early VATS for blunt chest trauma: a management technique underutilized by acute care surgeons. J Trauma 2011;71:102-5; discussion 105-7. [Crossref] [PubMed]
- Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr Opin Pulm Med 2015;21:393-8. [Crossref] [PubMed]
- Majercik S, Vijayakumar S, Olsen G, et al. Surgical stabilization of severe rib fractures decreases incidence of retained hemothorax and empyema. Am J Surg 2015;210:1112-6; discussion 1116-7. [Crossref] [PubMed]
- Chou YP, Kuo LC, Soo KM, et al. The role of repairing lung lacerations during video-assisted thoracoscopic surgery evacuations for retained haemothorax caused by blunt chest trauma. Eur J Cardiothorac Surg 2014;46:107-11. [Crossref] [PubMed]
- Truitt MS, Murry J, Amos J, et al. Continuous intercostal nerve blockade for rib fractures: ready for primetime? J Trauma 2011;71:1548-52; discussion 1552. [Crossref] [PubMed]
- Burlew CC, Davis KA, Fildes JJ, et al. Acute care surgery fellowship graduates' practice patterns: The additional training is an asset. J Trauma Acute Care Surg 2017;82:208-10. [Crossref] [PubMed]