Perioperative outcomes of radical lobectomies using robotic-assisted thoracoscopic technique vs. video-assisted thoracoscopic technique: retrospective study of 1,075 consecutive p-stage I non-small cell lung cancer cases
Introduction
The da Vinci Surgical System was first introduced to mainland China in 2006, and the first robotic-assisted pulmonary surgery was performed in Shanghai Chest Hospital in 2009. In the past decade, robotic-assisted surgeries have gained popularity all over China. Although three-arm robotic-assisted lobectomy (RAL3) (1) is theoretically better than video-assisted lobectomy (VAL) in 3-D visualization and improved maneuverability and ergonomics (2), apart from its higher cost, whether RAL3 is better than VAL in perioperative and long-term oncologic data is still under debate. Many studies have shown that RAL3 is comparable to VAL both in perioperative (3,4) and long-term oncologic data (5,6), but the majority of the previous studies are small cohorts meaning most of the surgeons are still in the early stage of the learning curve or META-analysis.
The purpose of this study was to see whether RAL3 is superior to VAL in early-stage lung cancer treatment in terms of perioperative outcomes. We reviewed over 1,000 consecutive patients who underwent lobectomies (237 RAL3 cases and 838 VAL cases) by the same surgical team (one surgeon and three assistants). Confounding factors were minimized using propensity score matching (PSM).
Methods
We retrospectively collected all the pathological stage I non-small cell lung cancer (NSCLC) cases from May 2013 to April 2016 done by the same surgical team, and 2502 cases (370 RAL3 cases, 1,708 VAL cases, and 124 open cases) were included. We ruled out the cases that directly received thoracotomy (124 cases), and the cases with more than one lobe resected (30 RAL3 cases, 102 VAL cases), sublobectomies (84 RAL3 cases, 991 VAL cases), lobectomies after prior pulmonary resections (14 RAL3 cases, 54 VAL cases), and lobectomies without LN sampling or dissection (5 RAL3 cases, 23 VAL cases). At last, 1,075 cases (237 RAL3 cases, 838 VAL cases) of p-stage I NSCLC patients were included for analysis. All data were retrieved from the medical records to assess patients’ and diseases’ characteristics [age, gender, height, weight, preoperative pulmonary function, and pre-operative American Society of Anesthesiologists (ASA) score], perioperative outcomes [operation time, estimated blood loss, number of dissected LNs, postoperative day 1 (POD1) chest tube drainage, duration of chest tube drainage and length of postoperative hospital stay]. All patients were staged based on the eighth edition TNM classifications of the International Association for the Study of Lung Cancer (IASLC). The study was approved by the Institutional Review Board of Shanghai Lung Tumor Clinical Medical Center, Shanghai Chest Hospital, Shanghai Jiao Tong University. All procedures were performed by one principal surgeon who had completed over 1,000 VAL lobectomies and 20 RAL3 lobectomies prior to this study.
Definitions
Forced expiratory volume in one second (FEV1) was used to assess preoperative pulmonary function. The operation time was defined as the time from skin incision was made to the closure of it (skin-to-skin time). POD1 drainage was defined as the drainage from POD1 6 A.M. to POD2 6 A.M. Conversion to thoracotomy, re-operation, and 30-day mortality was recorded. The cost was defined as the total cost during the in-hospital time. The length of postoperative stay for 95% patients was 2–8 days, so we defined PPHS (prolonged postoperative hospital stay) as the postoperative hospital stay over 8 days, and further analysis showed that the causes of PPHS were an air leak, pneumonia, hemothorax, chylothorax and bronchopleural fistula (BPF). The clinical diagnosis of pneumonia included X-ray chest radiographs suggesting new or progressive exudative lesions, combined with two of the three clinical manifestations (body temperature >38 °C, increased or decreased white blood cell (WBC) count, purulent sputum). Hemothorax was defined as hemorrhagic pleural effusion which was corrected with re-exploration, or conservative treatment like a blood transfusion or hemostasis management. Chylothorax was defined as pleural fluid triglyceride concentration >110 mg/dL (1.24 mmol/L) on a regular or high fat diet. The diagnosis of BPF is made using a combination of clinical, radiographic, and bronchoscopic findings that confirm an air leak from a major, lobar, or segmental bronchus to the pleural space. Re-operation was defined as an unexpected surgery within 30 days after the lobectomy.
Surgical technique
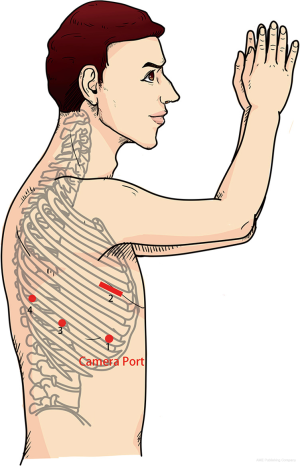
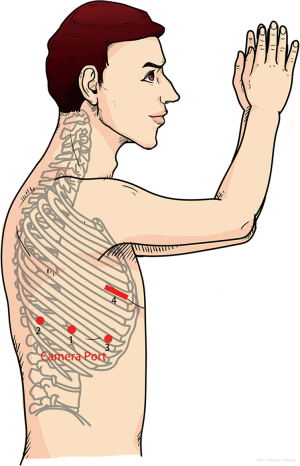
All procedures were performed with the patient in the lateral decubitus position, with anatomical removal of an entire lobe, along with mediastinal LN dissection which was a removal of ≥3 ipsilateral mediastinal (N2) lymph node (LN) stations in addition to removal of regional N1 LN stations for appropriate surgical staging of NSCLC. Rib-spreading technique was not used in either approach unless conversion to thoracotomy was needed under critical situations. VAL was performed using one 4 cm utility incision at the 4th intercostal space (ICS) anterior axillary line (3rd for upper lobectomy), one 12 mm camera port at 7th ICS anterior axillary line, one 12 mm incision at the 8th ICS posterior axillary line and one 1 2mm incision at the 8th ICS infrascapular line (Figure 1) (7). VAL usually needs one surgeon, and two assistants, one in charge of the thoracoscope and the other helping retract the lung and exposing the operating fields. RAL3 was performed using the da Vinci S Robotic Surgical System (Intuitive Surgical Inc., Mountain View, CA, USA), and using a three-port approach with a utility incision. Firstly, a 12 mm thoracoport was made at the 8th ICS posterior axillary line, then two 8 mm port incisions were symmetrically made at the 7th ICS mid-axillary line and the 9th ICS infrascapular line separately, and a 4 cm utility incision was made at the 4th ICS anterior axillary line (3rd ICS for right middle lobectomy) (Figure 2). Carbon dioxide was insufflated to a pressure of 8–10 mmHg. A 30-degree-angle-down stereoscopic camera was inserted through the camera port to explore the thoracic cavity. A Cadiere forceps and a cautery hook were manipulated by the left and right arm separately. The utility incision was used by the bedside assistant for retracting the lung, exposing the operating fields, and stapling and specimen retrieval.
For VAL, we adopted the unidirectional vein-bronchus-artery-sequence (vein-artery-bronchus for right upper lobectomy) thoracoscopic lobectomy described in 2010 by Dr. Liu et al. (8). For RAL3, we took an artery-bronchus-vein sequence to perform an upper lobectomy and the same vein-bronchus-artery sequence with VAL for middle and lower lobectomies.
RATS right upper lobectomy
A double joint oval clamp was inserted through the utility incision to grip the right upper lobe and tuck it cranially, and a suction tip was inserted to help further expose the operative fields at the same time. We usually started by completing the interlobar fissure and the pleura over the right upper bronchus to expose the arteries and bronchus, then dissect the interlobar LNs to expose branches of the pulmonary arteries further. A3 was the first structure to be sectioned using a mechanical stapler; then the lung was tucked ventrally to dissect and transect the bronchus and A1+2. Next, the lobe was tucked dorsally, and the vein and remaining fissure were finally transected. All staplers were introduced through the utility incision. A pair of dissecting forceps were introduced through the utility incision to achieve full dissection of the target structure when needed.
RATS right middle lobectomy
A double-joint oval clamp was inserted through the utility incision to grip the right upper lobe and tuck it cranially, while a suction tip was inserted to help further expose the operative. We usually started by completing the transverse and oblique fissure using a Cadiere forceps and a cautery hook. With the lung dorsally tucked, the pleura over the middle lobe vein was dissected, and the middle lobe vein itself was then dissected and transected. The two branches of the bronchus middle lobe artery were dissected and transected sequentially. The remaining fissure was at last transected. All staplers were introduced through the utility incision. A pair of dissecting forceps were introduced through the utility incision to achieve full dissection of the target structure when needed.
RATS right lower lobectomy
A double-joint oval clamp was inserted through the utility incision to grip the right upper lobe and tuck it cranially, while a suction tip was inserted to help further expose the operative fields. We started by completing the oblique fissure to expose the inferior pulmonary artery, and then dissected the pleura over the intermediate bronchus with the right lower lobe tucked ventrally. We then dissected the inferior pulmonary ligament, and inferior pulmonary vein with the lung lifted upright and transected the vein with the stapler. The bronchus and inferior pulmonary artery were dissected and transected sequentially. All staplers were introduced through the utility incision. A pair of dissecting forceps were introduced through the utility incision to achieve full dissection of the target structure when needed.
RATS left lung resections
This was completed in the same manner as the right lung resections. We usually took an artery-bronchus-vein sequence for upper lobectomy. Unlike the technique described by Pardolesi et al. (9), we used a vein-bronchus-artery sequence to do the left lower lobectomy, and all the stapling was done through the utility incision alone.
Postoperative management
All patients received postoperative analgesia with an analgesic pump, and those who were unsatisfied could receive intravenous or oral analgesia as needed. Chest tubes were blockaded for 24 hours when the output dropped below 300 mL, and there was no air leak after POD1. The chest tube could be removed if there was no fever, subcutaneous emphysema or pneumothorax, etc. Chest tube suction was applied in case of persistent air leak or poor lung re-expansion.
Statistical analysis
Patients receiving RAL3 and VAL were paired using PSM to minimize the bias between the two groups of patients. A nearest-neighbor matching method was adopted. After the PSM, 230 pairs of patients were matched based on the following covariates: age, gender, height, weight, tumor location (lobe), FEV1, ASA score, and tumor size on CT scan. The covariates like age, gender, height, weight, ASA score, and FEV1 were parameters that were used to describe the general status of the patients, and tumor location and tumor size were parameters that described the characteristics of tumors.
Comparison of means of continuous variables was conducted using the Student’s t-test (two-sided) and the nonparametric Mann-Whitney U test. Comparisons between binary and categorical variables were conducted using the chi-squared test. Fisher’s exact test was used to compare differences in proportions when expected numbers in any cell were less than 5 units. Results were considered statistically significant for Pvalues ≤0.05. STATA statistical software, v14.0 (StataCorp LP, College Station, TX, USA) was used for PSM, and SPSS software, v17.0 (SPSS Inc., Chicago, IL, USA) was used for further data analysis.
Results
From May 2013 to April 2016, a total of 1,075 p-stage I NSCLC patients received minimally invasive lobectomies and LN dissections, of whom 237 underwent RAL3 and 838 underwent VAL.
Patients’ and diseases’ characteristics
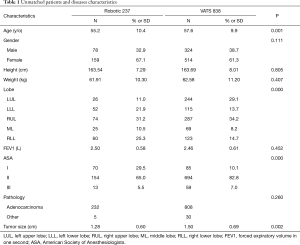
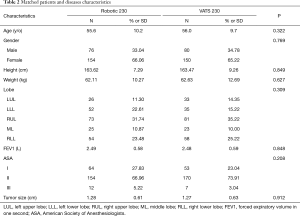
Patients’ and diseases’ characteristics are shown in Table 1. Before matching, patients receiving VAL and RAL3 had similar distribution for gender, height, weight, and FEV1, but had a notable difference for age, tumor location, ASA score, and tumor size. Patients receiving RAL3 were younger (55.2 vs. 57.6 yrs; P=0.001), in a better physical status (ASA I 29.5% vs. 10.1%; P=0.000) and with a smaller tumor size (1.28 vs. 1.50 cm; P=0.002). After matching, no obvious difference was observed between the two groups of patients as shown in Table 2.
Full table
Full table
Pre-PSM perioperative outcomes
The pre-PSM perioperative outcomes are shown in Table 3. The results showed that RAL3 was safer than VAL due to its lower rate of estimated blood loss (P=0.004) and less POD1 drain (P=0.001), but considering the key indicators for safety, which were conversion rate and reoperation rate, the advantage was not obvious. The patients receiving RAL3 had shorter chest tube duration (3.82 vs. 4.46 d, P=0.000) and postoperative length of stay (4.95 vs. 5.59 d, P=0.000). Both the retrieved LN stations and counts were more for the RAL3 group than the VAL group (P<0.001). The PPHS rate seemed lower for the RAL3 group compared to the VAL group (3.4% vs. 5.4%, P=0.206), while the skin-to-skin time seemed shorter for the RAL3 group (90.46 vs. 95.30 min, P=0.208). RAL3s had a higher cost than VALs (¥93,321.45 vs. ¥66,926.81, P=0.000). No readmission or death was observed within 30 days after discharge of the RAL3 group, while 2 patients in the VAL group were readmitted for BPF.
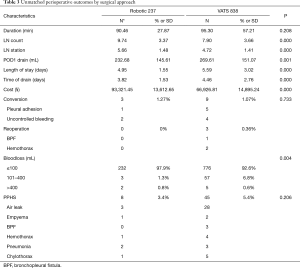
Full table
Post-PSM perioperative outcomes
The post-PSM perioperative outcomes are shown in Table 4. No significant difference was observed between the RAL3 and VAL group considering the average skin-to-skin time (90.84 vs. 92.25 min, P=0.624), conversion rate (1.3% vs. 0.87%, P=1.000) and PPHS rate (3.0% vs. 4.3%, P=0.694). Estimated blood loss of RAL3s during surgery was less than VALs (≤100 mL 225 vs. 216, 97.8% vs. 93.9%; 100–400 mL 3 vs. 13, 1.3% vs. 5.7%; >400 mL 2 vs. 1, 0.9% vs. 0.4%; P=0.035). No RAL3 patients required reoperation, while 2 VAL patients required reoperation for hemothorax. Compared to the VALs, the RAL3s had a higher retrieved LN (9.70 vs. 8.45, P=0.000) and stations count (5.66 vs. 4.72, P<0.001), less POD1 drain (230.91 vs. 279.79 mL, P=0.001), shorter chest tube duration (3.84 vs. 4.33 d, P=0.003) and shorter postoperative length of stay (4.97 vs. 5.45 d, P=0.004), but a higher cost (¥93,244.84 vs. ¥67,055.82, P=0.000). No readmission or death was observed within 30 days after discharge.
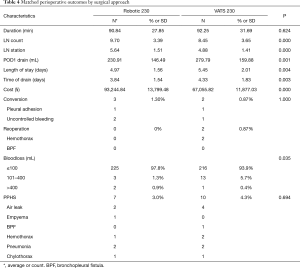
Full table
Discussion
In a recent, and by far the largest, meta-analysis comparing RATS and VAL in treating lung cancer, results showed that RATS is better than VAL in both 30-day mortality and conversion rate to open surgery, while no difference was observed in postoperative complications, operation time, duration of hospitalization, days to tube removal, or retrieved LN and retrieved LN station count (3). However, most of the included studies were small cohorts, which indicates that most RAL cases were probably done at an early phase of the learning curve compared to that of VAL. Unlike most of the previous studies, all the cases in the present study, apart from our 20 earliest RAL3 cases, were carried out by the same surgical team, and this is by far the largest single-surgical-team propensity score matched cohort study from mainland China.
Our results confirm that RAL3 is safer than VAL when considering its less intraoperative blood loss, shorter draining time, shorter postoperative length of stay, and comparable conversion and re-operation rate for early-stage NSCLC. Compared to VAL, the three-dimensional (3D) visualization and the improved maneuverability and ergonomics make it easier for RAL3 to identify the fine anatomical structures and handle them properly, thus reducing the chances of unexpected injury to the bronchial artery, lymphatic vessels, lung parenchyma, etc. These advantages can explain the less intraoperative blood loss, shorter draining time and shorter postoperative length of stay.
Our results also showed that both the retrieved LN and station counts were higher for the RAL3 group. The advantages mentioned above made it easier to dissect the LNs, especially the interlobar or intersegmental ones, which can explain the higher retrieved LN count for RAL3.
Many previous studies (10-12) have shown that RATS has a significantly longer operative time than VAL, which is mainly due to the inclusion of surgeons’ early experiences. With the exclusion of our first 20 cases, we found that the skin-to-skin operative time of RAL3 seems to be shorter than VAL. Instead of a complete four-port method (13) described by Cerfolio in 2011, we highly recommend a three-port method with a utility incision to shorten the total operative time, but unlike the three-port method described by Park in 2006 (7), we adopt a different method of port placement. In the four-port setting, the expansion of one existing port is always needed in the end for specimen retrieval, and different surgical instruments have to be frequently changed during the surgery, which significantly increases the operative time. Compared to the port incision, the utility incision allows for more than one surgical instrument to pass through at the same time, so that all the dissecting and transecting procedures can be done by the bedside assistant.
The vast majority of our perioperative results are similar to previous studies, but there are significant differences in conversion rate and operation time (3). As we mentioned before, most of the surgeons were still in the early phase of their learning curve. Even though we only excluded our first 20 cases, our conversion rate was still extremely low and the operation time was much shorter for both RAL3 and VAL. This remarkable difference may be mainly due to the surgeon’s experience in micro-invasive surgery, and also attributable to other factors like the race of the patients, region of the study and time of the study.
For now, the biggest disadvantage of RAL3 was its higher cost which was consistent with similar studies in the literature (4,14). However, we believe that with the advances in technology, the device-related cost could be greatly reduced in the near future.
Our research has limitations. First, it is a retrospective single-center study, and the PSM which was implemented could only decrease the selection bias, not eliminate it completely. Second, we only included patients of pathological stage I who had received lobectomy at the same time in order to minimize the confounding factors. However, for non-early stage NSCLC, or other types of lesions, this study has little practical significance. Third, the study lacks long-term oncology follow-up data. Finally, we did not describe the complications like other studies; we look forward to performing future prospective research comparing VAL and RATS with long-term follow-up data.
Conclusions
This study confirms that RAL3 is a safer and more effective technique than VAL for the treatment of early-stage NSCLC. Future studies should focus on the long-term benefits of RAL3 compared with VAL.
Acknowledgments
None.
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Shanghai Chest Hospital Affiliated Shanghai Jiao Tong University ethics committee [approval number: KS(P)1811].
References
- Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg 2017;154:1065-9. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic-assisted pulmonary resection-left lower lobectomy: how to do it. J Thorac Dis 2017;9:1658-62. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Bao F, Zhang C, Yang Y, et al. Comparison of robotic and video-assisted thoracic surgery for lung cancer: a propensity-matched analysis. J Thorac Dis 2016;8:1798-803. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Swanson SJ. Robotic pulmonary lobectomy--the future and probably should remain so. J Thorac Cardiovasc Surg 2010;140:954. [Crossref] [PubMed]