
CT-guided fine-needle aspiration biopsy of solitary pulmonary nodules under 15 mm in diameter: time for an afterthought?
Introduction
With technical advances in computed tomography (CT) technology, the growing use of multi-row detector CT and the diffusion of screening programs, we have observed a dramatic increase in the identification of small pulmonary lesion (1,2). Moreover, in the era of personalized medicine, the mutational pattern acquired great importance in the therapeutic strategy for lung cancer (3). The cytohistologic identification of these undetermined nodules, especially if small, could be a difficult step in the management of patients with suspected lung cancer. On the other hand, whether early detection of small malignant nodules could lead to a potentially curative surgical treatment, it is relevant to avoid unnecessary operations in the case of benign nodules (4,5).
CT-guided transthoracic fine needle aspiration biopsy (FNAB) is an established and safe technique for the diagnosis of intrathoracic lesions with a reported sensitivity that ranges from 74% to 95%, and a specificity between 87% and 100% (6-9). Several studies demonstrated that cell blocks (CBs) derived from FNAB can also provide sufficient material for the molecular profiling, such as EGFR mutation and ALK gene rearrangement tests, which are mandatory for the therapeutic choice in lung adenocarcinoma (10-12). Thanks to the advances of the molecular techniques, which enable the identification of wide spectrum of mutations also using scant tissues, cytological specimens can represent the biologic source for molecular testing, avoiding further invasive procedures (13).
Few studies have been published regarding the diagnostic performance and complication prevalence of FNAB in the evaluation of small lung nodules (<20 mm). These studies reported that the diagnostic accuracy tends to decrease with the size of the lesions, and that the prevalence of complications, such as pneumothorax and chest tube placement, tends to rise as the size of lesion decreases (7-9,14-16).
The aim of this study was to determine the accuracy and the diagnostic value of FNAB of solitary pulmonary nodules under 15 mm in diameter, in high risk patients.
Methods
This was an observational, retrospective, single-centre, cohort study conducted at Ca’ Granda Foundation University Hospital in Milan. The medical records of patients who were referred to our institution from January 2012 to December 2014 were reviewed.
Inclusion criteria were patients with SPN ≤15 mm in diameter and American Society of Anesthesiologists (ASA) status III, previous chest surgery (lung resections) who required cytological assessment with FNAB. Nodules with diameter of 3 cm or less, single, spherical, well-circumscribed, and surrounded by aerated lung were considered SPN (1,2,17). Study end-points were FNAB adequacy, diagnostic accuracy, complications prevalence and clinical value.
All biopsies were performed according to a standard protocol after a multidisciplinary evaluation by thoracic surgeons, oncologists and pathologists. Patients were previously instructed to discontinue anticoagulation therapy if they took it routinely. CT and PET scans, routinely performed before multidisciplinary evaluation, were reviewed immediately before the procedure. All FNABs were performed by a thoracic surgeon with large experience (Dr Tosi D). Informed consent was obtained from all patients before the procedure. The SPN diameter, its location (peripheral, mid-lobar or hilar), and the clinical outcome were extracted from patients’ charts.
At the time of biopsy, spiral CT scan was performed through the area of interest setting a slice thickness of 5 mm. The patient was then positioned on the CT table according to the best position for biopsy. Skin entry point was marked with a metallic marker and 10 mL of 2% lidocaine were administered to induce local anesthesia. Twenty-three Gauge Chiba needles were used in majority of the cases, but in case of lesions close to the chest wall, 22 Gauge needles were preferred, because of the limited risk of pneumothorax. The length of the needle was chosen according to the distance of the lesion from the chest wall. A step by step progression of the needle tip was then confirmed by CT scan (Figure 1). After confirmation of adequate position of the needle tip, aspirates were obtained; a part of the material was settled up with MGG (May-Grunwald-Giemsa) stain and submitted to a cytopathologist with experience in pulmonary pathology, in order to have a rapid on-site evaluation (ROSE). If the specimen was considered adequate, the procedure ended, if not, the aspirations were repeated up to three times.
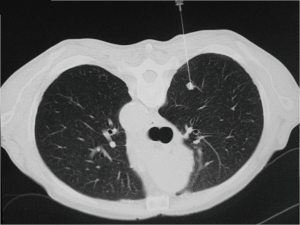
A final CT scan of the region of interest was performed to exclude complications, in particular pneumothorax. Chest tube was inserted immediately under CT guide if the pneumothorax was large or if the patient was symptomatic; smaller asymptomatic pneumothorax was treated by placing the patient in a recumbent position. All patients had an expiration chest X-ray in erect position one hour after the FNAB, in order to exclude pneumothorax or hemorrhage. Patients without complications were subsequently discharged with detailed instructions about possible late symptoms, while a second chest X-ray was obtained after one hour in patients with radiographic evidence of small pneumothorax. If the pneumothorax was “large” at one-hour chest X-ray, or when increasing pneumothorax has been documented on the second chest X-ray, the patients were treated with thoracostomy tube insertion. Symptomatic patients (shortness of breath, decreased oxygen saturation and substantial pain) had chest tube placement irrespectively to amount of pneumothorax (18).
The FNAB samples were fixed in formalin; samples were centrifuged in order to prepare paraffin blocks; immunohistochemistry was performed when an adequate specimen was available. According to well established criteria recommended by the World Health Organization (WHO 2004), the major histological criteria for adequate specimens are summarized.
- Squamous cell carcinomas: cohesive sheets and single cells with evidence of keratinization, Nucleus/Cytoplasm ratios range from low to high; dense, waxy, hard orangeophilic cytoplasm with clearly defined borders, cells with bizarre forms and hyperchromatic nuclei with inconspicuous or pyknotic nucleoli.
- Adenocarcinoma: cohesive sheets or clusters of cells with depth focus (three-dimensional); columnar, cuboidal, polygonal cells with scant/moderate cytoplasm with vacuolization, enlarged nuclei with prominent nucleoli.
- Small cell carcinoma: highly cellular aspirate with prominent coagulative necrosis and a high nucleus/cytoplasm ratio: very little cytoplasm and hyperchromatic nuclei with dispersed chromatin with a “salt and pepper” feature. In this subset of lesions, the research of neuroendocrine differentiation was performed with immunohistochemical analysis using the following antibodies: CD56, synaptophysin, and chromogranin A; furthermore, a proliferation index (Ki-67) was analyzed.
All the mentioned criteria were taken into consideration by the cytopathologist in association with clinical-instrumental data (anamnesis, radiological history, CT-PET), especially in patients with a low suspicion for malignancy.
The final cytological findings were classified positive for malignant tumor cells (MTC) or suspicious for malignancy. In this study MTC or “suspicious for malignancy” were considered as “positive” results. When there was evidence of a consistent cellular component but without any evidence of malignant tumor cells, the sample was recorded as negative for MTC; for the study aim, such findings were defined as a “negative” result after three punctures. We considered inadequate findings smears with insufficient or poor-quality cellular material from samples collected after three punctures. Patients with inadequate tests were submitted to surgical biopsy, repeated FNAB or clinical follow-up according to patients’ preference; such patients were excluded from the accuracy analysis (19,20).
Patients with positive results were considered for lung resection; patients with negative findings were followed up in order to exclude disease progression. After surgery, histological confirmation was obtained from formal fixed and paraffin embedded material when areas with squamous or glandular differentiation were observed. In poorly differentiated lesions, glandular differentiation was detected by TTF-1 and/or Napsin A immunohistochemical positivity as well as for P40 in squamous tumors or CD56 and/or synaptophysin for neuroendocrine differentiation.
Diagnostic accuracy was calculated by comparing the cytological diagnosis from the FNAB with findings obtained at surgical pathological examination or clinical follow-up.
Statistical analysis
Continuous data are presented as mean and standard deviation. Categorical variables are shown as numbers and percentages. Confidence interval for proportion was computed using normal approximation. Exact binomial confidence limits were calculated for sensitivity, specificity, and positive and negative predictive value (21). Confidence intervals for negative likelihood ratios were based on formulae provided by Simel (22). Chi square test was performed. Two-sided P values were computed. Statistical significance was considered when P value was equal or less than 0.05. Confidence interval was set at 95% confidence level. All analyses were carried out using R version 3.2.2 software (23).
Results
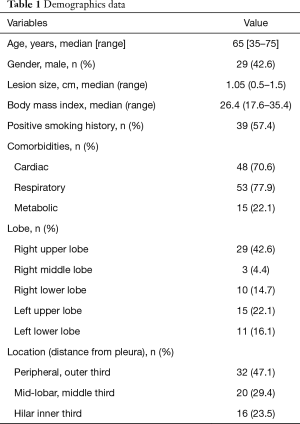
Out of 225 patients with SPN who were referred to our institution in the mentioned period, 68 matched the inclusion criteria. Demographics data are showed in Table 1. Chiba needles with a size of 23 Gauge were used in 85% of cases. Nineteen procedures (28%) were inadequate for diagnosis; 49 procedures resulted adequate and were considered for diagnostic accuracy (72%; 95% CI: 60–82%).
Out of the 49 adequate results, 35 were positive for malignant tumor cells and 14 were negative (1 case: tuberculosis). Among the 35 positive cases, 12 nodules (34%) were classified as “suspicious for malignancy” or non-small cell lung cancer (NSCLC), the remaining 23 cases had the histological type determined by immunohistochemistry: 9 (25%) adenocarcinomas, 8 (22%) squamous cell carcinomas, 2 well differentiated neuroendocrine tumors, 2 colorectal metastases, 1 uterine metastasis, and 1 bladder metastasis. Of the 35 cases positive for malignancy at FNAB, 33 patients had surgical confirmation (31 lung cancer and 2 pulmonary metastases). Two patients with lung metastases at FNAB findings were followed for more than 2 years with clinical confirmation; those patients were treated with chemotherapy or/and radiotherapy. Finally, out of the 49 adequate samples, 35 were recorded as true positives. Among the 14 negative cases, 5 nodules were specific benign lesions (4 granulomas, 1 hamartoma); in 4 cases inflammatory cells with a positive bacterial or mycobacterial culture were found. The remaining 4 cases had a nonspecific benign cytology (macrophages, histiocytes, reactive bronchial epithelium). The 14 patients with negative FNAB results were considered as true negative after follow-up without evidence of disease progression (median follow-up: 39 months; range, 25–59 months). Specificity was 100% (95% CI: 77–100%), sensitivity was 100% (95% CI: 90–100%). Diagnostic accuracy value was 100% (95% CI: 93–100%). Positive predictive value was 1.0 (95% CI: 0.9–1.0), negative predictive value was 1.0 (95% CI: 0.77–1.0). Number needed to diagnose was 1.0 (95% CI: 1.0–1.5). No differences were found, comparing the diagnostic accuracy for lesion size of 10 mm or less and for lesion size ranging from 11 to 15 mm (P>0.5). Regarding 19 patients with inadequate results from biopsy, every case was discussed during multidisciplinary team meetings, and submitted to surgical biopsy or radiologically followed up, depending on the clinical suspicious of malignancy.
The total overall number of punctures was 129; of them, 44 were the punctures in positive samples (mean: 1.25/patient), 42 in negative samples (mean: 3/patient) and 43 in inadequate samples (mean 2.26/patient). In the group of malignant cases, a single aspirate was sufficient for diagnosis in 27 cases (77%), a second puncture was required in 7 cases (20%), a third in 1 (3%).
The most frequent complication related to the FNAB procedure was pneumothorax, that occurred in 27 patients (39%, 95% CI: 28–52%); 19 patients required a chest drainage (28%). Pneumothorax occurrence and chest drain requirement were significantly associated with the number of punctures (P<0.001 and P<0.001, respectively). The association between respiratory comorbidities and pneumothorax rate is not statistically significant in our study. It is to underline that respiratory comorbidities, particularly emphysema is quite high in our cohort of patients (78%, as reported in Table 1), and this can be related to the lack of strong association. No other statistically significant risk factors were found; there was a trend of higher prevalence of pneumothorax rate in the group of patients with “inner third” lesions, but this wasn’t statistically significant, probably due to the small size of such subgroup.
Full table
There was one case of hemoptysis, with spontaneous resolution after a short time without any treatment; no cancer cell seeding occurred in this series.
Discussion
The increased availability of CT with improved technology (such as sub-millimeter CT scan) has increased the detection rate of small pulmonary nodules, including early peripheral lung cancer (1,2). The recent diffusion of cancer screening programs makes the management of small pulmonary nodules a challenging issue. Furthermore, many more patients undergo screening autonomously. Finally, current targeting therapies require tissue sampling for molecular tests. Percutaneous fine needle biopsy of the lung has over the years become a simple, quick, safe and highly accurate procedure in the clinical management of patients with pulmonary lesions, especially the solitary ones. FNAB is an alternative to video-assisted thoracoscopic surgery in the diagnosis of solitary pulmonary nodules (17). Despite significant percentage of non-diagnostic results, FNAB technique is less invasive than VATS, avoids general anesthesia, and is associated with a lower rate of complications. Considering such characteristics, FNAB is usually suggested in high risk patients (3-8).
Several studies had fixed the FNAB overall sensitivity at 70–100% for the diagnosis of malignancy, and most reported values range from 85% to 95% (6-9,14-16,24,25). The same diagnosis could be obtained by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for peripheral lesions, with lower complication rate, but lower sensitivity as well (26); for this reason, we still consider EBUS-TBNA as the gold standard in case of nodal involvement (27), but not as the first choice in case of peripheral lung cancer.
The issue about the possible role of the dimensions of the pulmonary lesions in terms of sensitivity, diagnostic accuracy and rate of complications is still open (14). Ohno et al. reported a diagnostic accuracy of 52% for lesion size of 10 mm or less and 74.4% for lesion size ranging from 11 to 15 mm (15). Wallace et al. reported an overall sensitivity and a diagnostic accuracy of 82% and 88% respectively in their series of FNAB of nodules ≤10 mm (16). In our study, the prevalence of adequate samples was 72%, a rate similar to Wallace (16). Interestingly, in our series the diagnostic accuracy for lesion size of 10 mm or less and for lesion size ranging from 11 to 15 mm was similar. It is well known that adequacy depends on the location, depth, diameter of the nodule and on the number of punctures; in addition, high cancer prevalence in a study population increases the adequacy (28). We consider adequacy prevalence over 70% a good reason for use FNAB as a suitable alternative to surgical biopsy in high risk patients. Nevertheless, the previous considerations should be carefully evaluated in every clinical case: an accurate selection of patients to submit to FNAB could potentially increase the adequacy up to 80%, as indicated by the upper limit of 95% confidence interval in our study.
Including inadequate findings in the accuracy calculation is a discussed matter; usually authors reporting FNAB on suspected breast lesions considered the inadequate results as malignant (29). From a clinical point of view, this behavior increases the indications to surgical biopsies, but it is obvious that surgical risk for pulmonary resection is considerable higher than breast surgery. Therefore, we don’t consider justified including inadequate samples as positive findings in the accuracy calculation, as well as in the clinical practice (19,20). On the other hand, some Authors included inadequate findings into negative results (28). Once again, we consider this assumption as a questionable clinical conduct: inadequate results should lead to repeat FNAB or consider further diagnostic strategy such as navigation bronchoscopy (30), surgical biopsy or close follow-up. From a methodological perspective, is it logical considering an inadequate finding a positive or negative result? According to other authors, we believe that inadequate samples can’t be included in the accuracy calculation (20,31,32).
In our experience about FNAB of small pulmonary lesions (≤15 mm), sensitivity and diagnostic accuracy value is considerable, especially if compared with the results on bigger nodules. This good result can be partially explained by analyzing the nature of the lesions in terms of CT appearance. Performing a biopsy of a small nodule is technically challenging, due to the difficulty in reaching the small target area. In the same way it is possible to state that biopsy in a large lesion is sometimes disheartening for the presence of a large necrotic area, which frequently leads to inadequate results. This consideration allows understanding how, although dealing with a small lesion is technically complicated, it often gets good results in terms of diagnostic accuracy.
During FNAB procedures, a cytopathologist should be present to reduce the number of non-conclusive specimens and decrease the number of recurrent biopsies (24,25,33-38). Moreover, in case of a malignant lesion, the cytopathologist can require an additional sample to be kept in formalin, in order to have sufficient material for immunohistochemical studies (39-43). In addition, the use of CBs obtained from FNAB for the molecular testing of the tumor, is essential for the benefit of the patient, whose treatment may depend on the EGFR and ALK status (3,44). The discussion of the clinical case with the cytopathologist can help to guide the strategy for the biopsy: for example, in the case of suspected granulomatous pathology, or in case of difficult malignancies, is possible to perform a tru-cut biopsy for the confirmation of the diagnosis (45,46). Core-needle biopsies have high diagnostic yield, with an acceptable increased complication rate, but were not included in the present study (47).
Although the estimated accuracy was extremely high in our study, its value must be cautiously considered: the lower limit of 95% CI indicates that, repeating the experience in a different scenario, the specificity and sensitivity should be at least 77% and 90%, respectively. Such values are similar to the performance obtained from the FNAB for larger SPNs. The intrinsic variability of FNAB could also be related to the pathological judgment. In this context, our selectiveness in determining sample adequacy and patients’ selection have led to a high diagnostic accuracy.
Among the possible complications, pneumothorax is by far the most frequent; its reported rate ranges widely from 22% to 45% (48-50). Cox et al. demonstrated, with a study about the variables that affected the risk of pneumothorax during FNAB, how the only variables that have proved to be statistically significant were the dimensions of the nodules and the evidence of emphysema (51). In a meta-analysis published in 2017 by Heerink et al., smaller nodule diameter, larger needle diameter and increased traversed lung parenchyma were risk factors for complications (52). In our series the pneumothorax prevalence was similar to that reported in the literature; we found an association between the number of punctures and pneumothorax rate as reported by others (53).
The possibility of cancer cells seeding is well known as a potential risk of FNAB; we did not find any cancer seeding in our series but, although the incidence is quite rare, the seeding must be taken in account in the follow up (54).
Study limitations were related to the small sample size and to a possible selection bias.
Conclusions
We believe that FNAB is a suitable tool for the diagnosis of lung nodule of 15 mm or less in high risk patients. Accurate patients’ selection, expert operator, rapid on-site evaluation and selectiveness in adequacy judgment are essential to reach high diagnostic accuracy that may avoid unnecessary surgical procedures. In fact, in our series, out of 49 patients who received adequate diagnosis, 14 (29%) of them resulted true negative and didn’t need surgical diagnosis.
In conclusion our study demonstrated that FNAB is an effective procedure even in the diagnosis of small pulmonary nodules. The accuracy resulted satisfactory while positive and negative predictive values reached the top rate. In the era of cancer screening and target medicine a diagnostic strategy including FNAB as the first approach to small pulmonary nodule in “sub-critical” patients should be revaluated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approved by Ethics committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano (2369/2012) and informed consent was obtained.
References
- Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest 2003;123:89S-96S. [Crossref] [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828-60. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;3:131S-48S.
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;3:94S-107S.
- Hayes MM, Zhang DY, Brown W. Transthoracic fine needle aspiration biopsy cytology of pulmonary neoplasms. Diagn Cytopathol 1994;10:315-9. [Crossref] [PubMed]
- Weisbrod GL. Transthoracic percutaneous lung biopsy. Radiol Clin North Am 1990;28:647-55. [PubMed]
- Larscheid RC, Thorpe PE, Scott WJ. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest 1998;114:704-9. [Crossref] [PubMed]
- Swischuk JL, Castaneda F, Patel JC, et al. Percutaneous transthoracic needle biopsy of the lung: review of 612 lesions. J Vasc Interv Radiol 1998;9:347-52. [Crossref] [PubMed]
- Billah S, Stewart J, Staerkel G, et al. EGFR and KRAS mutations in lung carcinoma: Molecular testing by using cytology specimens. Cancer Cytopathol 2011;119:111-7. [Crossref] [PubMed]
- Cai G, Wong R, Chhieng D, et al. Identification of EGFR mutation, KRAS mutation, and ALK gene rearrangement in cytological specimens of primary and metastatic lung adenocarcinoma. Cancer Cytopathol 2013;121:500-7. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Maxfield RA, et al. Molecular testing guidelines for lung adenocarcinoma: Utility of cell blocks and concordance between fine-needle aspiration cytology and histology samples. Cytojournal 2014;11:12. [Crossref] [PubMed]
- Kriegsmann M, Arens N, Endris V, et al. Detection of KRAS, NRAS and BRAF by mass spectrometry - a sensitive, reliable, fast and cost-effective technique. Diagn Pathol 2015;10:132. [Crossref] [PubMed]
- Li H, Boiselle PM, Shepard JO, et al. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996;167:105-9. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. [Crossref] [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Kalanjeri S, Holladay RC, Gildea TR. State-of-the-Art Modalities for Peripheral Lung Nodule Biopsy. Clin Chest Med 2018;39:125-38. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Silva WP, Stramandinoli-Zanicotti RT, Schussel JL, et al. Accuracy, Sensitivity and Specificity of Fine Needle Aspiration Biopsy for Salivary Gland Tumors: A Retrospective Study from 2006 to 2011. Asian Pac J Cancer Prev 2016;17:4973-6. [PubMed]
- Georgescu R, Oprea AL, Contra A, et al. The sensitivity and specificity of fine-needle aspiration in thyroid neoplasia. Journal of Interdisciplinary Medicine 2017;2:127-31. [Crossref]
- Collett D. Modelling Binary Data. Boca Raton, Florida: Chapman & Hall/CRC, 1999;24.
- Simel DL, Samsa G, Matchar D. Likelihood ratios with confidence: Sample size estimation for diagnostic test studies. J Clin Epidemiol 1991;44:763-70. [Crossref] [PubMed]
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2015. ISBN 3-900051-07-0.
- Santambrogio L, Nosotti M, Bellaviti N, et al. CT-guided fine-needle aspiration cytology of solitary pulmonary nodules: a prospective, randomized study of immediate cytologic evaluation. Chest 1997;112:423-5. [Crossref] [PubMed]
- Chang YC, Yu CJ, Lee WJ, et al. Imprint cytology improves accuracy of computed tomography-guided percutaneous transthoracic needle biopsy. Eur Respir J 2008;31:54-61. [Crossref] [PubMed]
- Zhan P, Zhu Q, Miu Y, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23-34. [Crossref] [PubMed]
- Rosso L, Ferrero S, Mendogni P, et al. Ten-year experience with endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal lymph-nodes. J Thorac Dis 2017;9:S363-9. [Crossref] [PubMed]
- Busso M, Sardo D, Garetto I, et al. Safety and diagnostic performance of image-guided lung biopsy in the targeted therapy era. Radiol Med 2015;120:1024-30. [Crossref] [PubMed]
- Yu YH, Wei W, Liu JL. Diagnostic value of fine-needle aspiration biopsy for breast mass: a systematic review and meta-analysis. BMC Cancer 2012;12:41. [Crossref] [PubMed]
- Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. [Crossref] [PubMed]
- Khalbuss WE, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1114 cases with cytological-histological correlation. Cancer Cytopathol 2010;118:24-32. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Mazza E, Maddau C, Ricciardi A, et al. On-site evaluation of percutaneous CT-guided fine needle aspiration of pulmonary lesions. A study of 321 cases. Radiol Med 2005;110:141-8. [PubMed]
- Padhani AR, Scott WW Jr, Cheema M, et al. The value of immediate cytologic evaluation for needle aspiration lung biopsy. Invest Radiol 1997;32:453-8. [Crossref] [PubMed]
- Baram D, Garcia RB, Richman PS. Impact of rapid on-site cytologic evaluation during transbronchial needle aspiration. Chest 2005;128:869-75. [Crossref] [PubMed]
- Fassina A, Corradin M, Zardo D, et al. Role and accuracy of rapid on-site evaluation of CT-guided fine needle aspiration cytology of lung nodules. Cytopathology 2011;22:306-12. [Crossref] [PubMed]
- Austin JH, Cohen MB. Value of having a cytopathologist present during percutaneous fine-needle aspiration biopsy of lung: report of 55 cancer patients and metaanalysis of the literature. AJR Am J Roentgenol 1993;160:175-7. [Crossref] [PubMed]
- Stewart CJ, Stewart IS. Immediate assessment of fine needle aspiration cytology of lung. J Clin Pathol 1996;49:839-43. [Crossref] [PubMed]
- Zudaire I, Lozano MD, Vazquez MF, et al. Molecular characterization of small peripheral lung tumors based on the analysis of fine needle aspirates. Histol Histopathol 2008;23:33-40. [PubMed]
- Kim DH, Kwon MS. Role of fine needle aspiration cytology, cell block preparation and CD63, P63 and CD56 immunostaining in classifying the specific tumor type of the lung. Acta Cytol 2010;54:55-9. [Crossref] [PubMed]
- Khayyata S, Yun S, Pasha T, et al. Value of P63 and CK5/6 in distinguishing squamous cell carcinoma from adenocarcinoma in lung fine-needle aspiration specimens. Diagn Cytopathol 2009;37:178-83. [Crossref] [PubMed]
- Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol 2007;15:415-20. [Crossref] [PubMed]
- Fassina A, Gazziero A, Zardo D, et al. Detection of EGFR and KRAS mutations on trans-thoracic needle aspiration of lung nodules by high resolution melting analysis. J Clin Pathol 2009;62:1096-102. [Crossref] [PubMed]
- Lian W, Ouyang Y. CT-guided aspiration lung biopsy for EGFR and ALK gene mutation analysis of lung cancer. Oncol Lett 2017;13:3415-22. [Crossref] [PubMed]
- Greif J, Marmur S, Schwarz Y, et al. Percutaneous core cutting needle biopsy compared with fine-needle aspiration in the diagnosis of peripheral lung malignant lesions: results in 156 patients. Cancer 1998;84:144-7. [Crossref] [PubMed]
- Aviram G, Greif J, Man A, et al. Diagnosis of intrathoracic lesions: are sequential fine-needle aspiration (FNA) and core needle biopsy (CNB) combined better than either investigation alone? Clin Radiol 2007;62:221-6. [Crossref] [PubMed]
- Marchianò AV, Cosentino M, Di Tolla G, et al. FNA and CNB in the diagnosis of pulmonary lesions: a single-center experience on 665 patients, comparison between two periods. Tumori 2017;103:360-6. [Crossref] [PubMed]
- Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol 2003;58:791-7. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809-14. [Crossref] [PubMed]
- Halloush RA, Khasawneh FA, Saleh HA, et al. Fine needle aspiration cytology of lung lesions: a clinicopathological and cytopathological review of 150 cases with emphasis on the relation between the number of passes and the incidence of pneumothorax. Cytopathology 2007;18:44-51. [Crossref] [PubMed]
- Cox JE, Chiles C, McManus CM, et al. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology 1999;212:165-8. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Ayyappan AP, Souza CA, Seely J, et al. Ultrathin fine-needle aspiration biopsy of the lung with transfissural approach: does it increase the risk of pneumothorax? AJR Am J Roentgenol 2008;191:1725-9. [Crossref] [PubMed]
- Scotti V, Di Cataldo V, Falchini M, et al. Isolated chest wall implantation of non-small cell lung cancer after fine-needle aspiration: a case report and review of the literature. Tumori 2012;98:126e-9e. [Crossref] [PubMed]


