
Point-of-care coagulation management during surgery with minimal invasive extracorporeal circulation
Introduction
Contemporary advances in cardiac surgery have markedly improved clinical outcomes. However, cardiac surgery is still hampered by considerable morbidity and subsequent mortality, especially in complex and high-risk procedures, as evidenced in the largest cardiac surgery registry (1). A strong contributing factor is postoperative coagulation derangement mainly due to the use of cardiopulmonary bypass (CPB). The resultant acute postoperative bleeding is a life-threatening major resource-consuming complication that impairs outcome (2).
The development of cardiac anesthesia contributed major progress to postoperative hemostasis via the integration of point-of-care (POC) monitoring of coagulation. A contemporary POC laboratory in the operating room (OR) includes thromboelastography (TEG, Haemonetics) or rotational thromboelastometry (ROTEM, TEM Innovations), aggregometry, and individualized heparin management (HMS, Medtronic) for precise titration of heparin and protamine (Table 1). The implementation of POC monitoring during cardiac surgery has significantly expanded our knowledge on the pathophysiology of coagulation derangement intraoperatively and led to advanced targeted treatment.

Full table
The development of POC coagulation management coincided with an evolution in perfusion science which was the establishment of minimal invasive extracorporeal circulation (MiECC) in clinical practice. MiECC represents a cutting-edge perfusion technology that integrates all contemporary advancements in perfusion in a closed circuit with biologically inert blood contact surfaces; this offers enhanced biocompatibility and provides optimal perfusion, which ultimately better preserves end-organ function (3). Our department is a leading institution in the field of MiECC research and technology that is routinely applied to all types of cardiac surgical procedures (4). We implement MiECC use in the context of a multidisciplinary perioperative strategy for attaining “more physiologic” cardiac surgery, as presented earlier in this focused issue (5).
Effect of MiECC on coagulation integrity
It is widely accepted that CPB represents a “non-physiologic” intervention since it perturbs the physiologic milieu in many aspects. The net effect of this systematic disarray is the sub-optimal or impaired intraoperative perfusion resulting in end-organ damage (6). This contributes to the inevitable postoperative morbidity and mortality observed in cardiac surgery. As mentioned, coagulation derangement contributes to this outcome.
The activation of coagulation pathway during conventional CPB induces a combination of thrombocytopenia and platelet dysfunction following contact with an artificial surface or by surgical trauma (7). On the other hand, MiECC can serve as a coagulation-preserving circuit (8) by comprising certain components: a closed circuit that eliminates blood-air interaction, biologically inert blood contact surfaces, reduced priming volume that minimizes hemodilution, a centrifugal pump that reduces fibrinolysis, and a shed blood management device arising from the absence of cardiotomy suction (3). The progression of MiECC from I to type IV design can be characterized by the overcoming of all safety concerns and unexpected intraoperative scenarios in the cardiac OR, and thus the establishment of compatibility with any cardiac case-mix (4).
The MiECC’s unique intrinsic characteristics for preserving hematologic integrity clinically translate to better preservation of hematocrit and reduction in the need for perioperative blood transfusion (class IA) (3). These results forced the EACTS/EACTA Task Force to integrate MiECC as an intraoperative strategy for the maintenance of hemostasis and blood conservation management in adult cardiac surgery (9). Moreover, MiECC allows for the adoption of a low-dose anticoagulation protocol in coronary procedures (10), hence reducing the adverse effects of heparin and protamine use.
Heparin and protamine titration
Traditionally, in the absence of a POC device for the precise titration of heparin and protamine, the estimation of a heparin dose is based exclusively on body weight. A target ACT value of 480 sec is set for all cardiac procedures. This empirical approach completely ignores the variability in heparin activity (related to the manufacturer), patients’ response to a certain dose (different antithrombin III levels), as well as other factors, such as bioavailability in circulation, rate of heparin metabolism and overt thrombin generation. Moreover, ACT values can be affected by hemodilution, hypothermia, platelet dysfunction, clotting factor deficiency and pH derangement. The unique characteristics of the MiECC circuit (minimal hemodilution, better biocompatible surfaces, elimination of blood-air interaction, exclusion of unprocessed shed blood reinfusion) favourably influence thrombin generation and allow for the application of a low anticoagulation protocol in cardiac surgery. Thus, an ACT value exceeding 300 sec for coronary surgery and 400 for valve and complex procedures operating on MiECC is considered adequate and safe. Individualized heparin management and heparin level-guided protamine titration, as recommended in the joined EACTS/EACTA guidelines, determines patient-specific heparin response, detects heparin resistance, and accurately calculates protamine dose that matches actual heparin levels (9). Ruling out residual heparin with POC testing instead of blind supplementary protamine administration is important because protamine overdose has an anticoagulation effect and may worsen the ongoing bleeding (11).
Despite this knowledge, although avoidance of protamine overdosing is of utmost importance in preserving coagulation integrity after termination of CPB, this notion has been overlooked by most cardiac anesthesiologists. Protamine neutralizes heparin, yet it exerts significant anticoagulant activity. Exposure to protamine may result in thrombocytopenia and reduced thrombin-induced platelet aggregation and function which is dose-dependent (12,13). This effect may be minimized by adaptation of the protamine dose to the residual heparin concentration at the end of CPB. According to Boer et al. protamine administration based on the initial heparin dose should target a ratio below 1:1 in order to prevent protamine-related coagulopathy and postoperative bleeding (11). The exact ratio is not elaborated and may vary between 0.6 and 1.0 based on POC heparin management. In general, application of a patient-specific anticoagulation protocol combined with a MiECC circuit results in a decrease in the total dose of protamine and in the preservation of patients’ hemostatic reserves.
Viscoelastic monitoring—thromboelastometry
Cardiac surgery decreases the activity of all coagulation factors. Among these, fibrinogen has been shown to significantly contribute to clot stability; accordingly, in one study, fibrinogen levels were associated with postoperative blood loss (14). Viscoelastic measurements assess the overall activity of coagulation factors and the interaction between platelets and fibrinogen. They provide a graphic representation of hemostasis kinetics (15). The growing viscoelasticity of the coagulating blood is continuously measured by sensors and finally visualized as a real-time graphical curve. Viscoelastic tests use whole blood and specific activators for intrinsic and extrinsic pathways (Table 1). The main parameters obtained from thromboelastometry are shown in Table 1.
Platelet function testing—aggregometry
Considering the increasing proportion of patients that receive dual antiplatelet medication, it has become evident why perioperative platelet dysfunction is currently considered one of the main causes of bleeding in the early period following cardiac surgery (16). Management in clinical practice relies solely on multiple platelet transfusions although there are not widely accepted evidence-based indications. Thus, platelet transfusion is considered empirical with a frequency that varies greatly among different institutions (17). It should be noted that platelet transfusion may be ineffective due to refractoriness, which is defined as the repeated failure to achieve satisfactory responses to platelet transfusions from random donors, which increases the risk for adverse outcomes (18). With the currently available aggregometers we can evaluate three different pathways of aggregation in response to stimulation (19), with three specific receptor agonist reagents (Table 1). Moreover, according to Ranucci et al. the function of adenosine diphosphate (ADP) and protease-activated receptor (PAR) receptors significantly predict the requirements for perioperative blood transfusion (20).
POC coagulation management
Algorithms based on POC testing have been proposed to ease assessment of a patient’s coagulation status and allow for a more specific coagulation therapy (21). We advocate individualized heparin and protamine management. Low anticoagulation protocols are used due to the use of MiECC systems, while continuous intravenous heparin infusion is preferred instead of intermittent heparin administration by boluses. A heparin-protamine ratio of 0.7 is used. Residual heparin levels are excluded before the patient leaves from the OR.
Despite routine use in our institution, POC coagulation management in the OR should be initiated when ongoing microvascular bleeding is observed in the surgical field after heparin reversal and exclusion of surgical hemorrhage. In cases where the presence of certain factors, such as hypothermia and/or emergency surgery could severely impair coagulation, thromboelastometry allows for the assessment of a patient’s coagulation status even in the presence of full heparinization before weaning from CPB, targeting a reduced bleed-to-treat time. In case of fibrinogen deficiency, fibrinogen concentrate or an appropriate amount of fresh-frozen plasma (FFP) or cryoprecipitate is administered as first line treatment. Prothrombin complex concentrates are administered as first line in the case of a significantly prolonged clotting time without evidence of residual heparin or fibrinogen deficiency, or as a second line in the case of a prolonged clotting time after fibrinogen/FFP supplementation.
In the event of ongoing microvascular bleeding after heparin reversal with titrated protamine and in presence of adequate fibrinogen levels, aggregometry tests should be evaluated. In the case of low values in at least two aggregometry tests, preferably ADP and TRAP tests, we consider platelet transfusion. We do not advocate desmopresin administration for improving platelet function, because it has no proven efficacy and also carries the risk of hemodynamic impairment.
The value of POC coagulation management during surgery with MiECC
As reflected in the published EACTS/EACTA guidelines, postoperative bleeding management is composed of a multidisciplinary approach based on the close collaboration between cardiac surgeons, anesthesiologists, clinical perfusionists, and intensivists (Pagano) (Figure 1). Current evidence shows that POC hemostatic tests reduce the rates of blood and platelet transfusions along with the incidence of major bleeding without affecting complication rates (22). This is the core of our institutional strategy. POC management, per se, can only “correct rather than prevent” the deleterious effects of CPB on coagulation cascade. On the other hand, MiECC technology in clinical practice obviates all deleterious effects of CPB on coagulation integrity, and can thus be considered a pinnacle development in this area of cardiac surgery. MiECC is considered an indispensable tool in performing a “more physiologic” cardiac surgery (23).
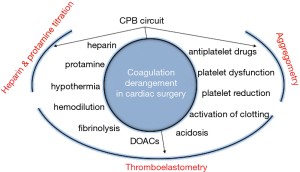
Applying POC heparin-protamine and coagulation management when using MiECC in combination with continuous transesophageal echocardiography and in-line intraoperative monitoring, upgrades MiECC from a system to a procedure and defines the so called “MiECC strategy” (5). This multidisciplinary approach is translated into a “prevent rather than correct” perioperative management, through preservation of metabolic, coagulation and microcirculatory integrity that ultimately leads to improved end-organ protection and an optimal clinical outcome.
All published literature on POC hemostasis monitoring is based on patients operated on with conventional CPB without individualized heparin/protamine management. Our institution is considered up-to-date and the only cardiac center that routinely applies both MiECC and POC monitoring in all operated patients. The reported cumulative benefits from the application of MiECC in reducing hemodilution and preserving hemostatic reserves ultimately result in improved clot quality. A further consequence of this is a significantly reduced need for blood product transfusion, a fact which becomes evident even after the first few cases (24). By analyzing original unpublished data obtained from heparin management and POC coagulation monitoring during surgery with MiECC, we managed to elicit significant preliminary results that provide an insight into the protective effect of MiECC in hemostasis. Thus, MiECC greatly facilitates implementation of low-anticoagulation protocols, and creates an ideal, stable and biocompatible environment for continuous heparin administration.
Ranucci et al. showed that in the cardiac surgery setting, hemodilution after CPB shifted patients’ profiles towards a hypercoagulable state which resulted in a higher rate of bleeding-related events (25). We noticed that the beneficial effect of MiECC due to avoidance of hemodilution and coagulation preservation results in a satisfied clot quality after CPB which is reflected in a very low FFP and platelet transfusion rate. Moreover, unpublished data from our registry show preservation of ADP receptors combined with improved function of PAR receptors related to preoperative values. This comes in overt contrast with the existing literature, which recommends the deterioration in platelet function as a rule in cardiac surgery when using conventional CPB (26). However, we support the notion proposed by Di Dedda et al. who assert that the preserved platelet function reflects a well-functioning microcirculation, which is probably the rationale behind the protective effect of MiECC on platelets (27).
Conclusions
POC heparin-protamine and coagulation monitoring is essential for the development of specific algorithms to diagnose and, consequently, treat perioperative bleeding in cardiac surgery. MiECC offers the basis for creating a strategy that leads to perioperative preservation of hemodynamic, metabolic and coagulation integrity even in complex and high-risk procedures by ameliorating the detrimental effects of conventional CPB. Implementing POC management during surgery with MiECC contributes to a more “physiologic” cardiac surgery that ultimately leads to improved end-organ protection and reduction in complications rate. Therefore, we advocate this strategy as the state-of-the-art technique in performing cardiac surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- D'Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons adult cardiac surgery database: 2018 update on outcomes and quality. Ann Thorac Surg 2018;105:15-23. [Crossref] [PubMed]
- Ranucci M, Baryshnikova E, Castelvecchio S, et al. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg 2013;96:478-85. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Argiriadou H, et al. Modular minimally invasive extracorporeal circulation systems; can they become the standard practice for performing cardiac surgery? Perfusion 2015;30:195-200. [Crossref] [PubMed]
- Anastasiadis K, Argiriadou H, Deliopoulos A, et al. Minimal invasive extracorporeal circulation (MiECC): the state-of-the-art in perfusion. J Thorac Dis 2019;11 Suppl 10:S1507-14.
- Murphy GS, Hessel EA 2nd, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009;108:1394-417. [Crossref] [PubMed]
- Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med 2004;30:1873-81. [Crossref] [PubMed]
- Anastasiadis K, Asteriou C, Deliopoulos A, et al. Haematological effects of minimized compared to conventional extracorporeal circulation after coronary revascularization procedures. Perfusion 2010;25:197-203. [Crossref] [PubMed]
- Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:79-111. [Crossref] [PubMed]
- Bauer A, Diez C, Schubel J, et al. Evaluation of hemodynamic and regional tissue perfusion effects of minimized extracorporeal circulation (MECC). J Extra Corpor Technol 2010;42:30-9. [PubMed]
- Boer C, Meesters MI, Veerhoek D, et al. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth 2018;120:914-27. [Crossref] [PubMed]
- Lindblad B, Wakefield TW, Whitehouse WM Jr, et al. The effect of protamine sulfate on platelet function. Scand J Thorac Cardiovasc Surg 1988;22:55-9. [Crossref] [PubMed]
- Ammar T, Fisher CF. The effects of heparinase 1 and protamine on platelet reactivity. Anesthesiology 1997;86:1382-6. [Crossref] [PubMed]
- Ternström L, Radulovic V, Karlsson M, et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res 2010;126:e128-33. [Crossref] [PubMed]
- Baryshnikova E, Ranucci M. Point-of-care haemostasis and coagulation monitoring in cardiac surgery at IRCCS Policlinico San Donato. Eur Heart J Suppl 2016;18:E42-8. [Crossref] [PubMed]
- Solomon C, Hartmann J, Osthaus A, et al. Platelet concentrates transfusion in cardiac surgery in relation to preoperative point-of-care assessment of platelet adhesion and aggregation. Platelets 2010;21:221-8. [Crossref] [PubMed]
- Bracey AW, Grigore AM, Nussmeier NA. Impact of platelet testing on presurgical screening and implications for cardiac and noncardiac surgical procedures. Am J Cardiol 2006;98:25N-32N. [Crossref] [PubMed]
- Kerkhoffs JL, Eikenboom JC, van de Watering LM, et al. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008;48:1959-65. [Crossref] [PubMed]
- Schimmer C, Hamouda K, Sommer SP, et al. The predictive value of multiple electrode platelet aggregometry (multiplate) in adult cardiac surgery. Thorac Cardiovasc Surg 2013;61:733-43. [Crossref] [PubMed]
- Ranucci M, Colella D, Baryshnikova E, et al. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth 2014;113:970-6. [Crossref] [PubMed]
- Weber CF, Gorlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012;117:531-47. [Crossref] [PubMed]
- Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation 2016;134:1152-62. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Deliopoulos A, et al. A multidisciplinary perioperative strategy for attaining "more physiologic" cardiac surgery. Perfusion 2017;32:446-53. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Asteriou C, et al. Quantification of operational learning in minimal invasive extracorporeal circulation. Artif Organs 2017;41:628-36. [Crossref] [PubMed]
- Ranucci M, Baryshnikova E, Ciotti E, et al. Hemodilution on cardiopulmonary bypass: thromboelastography. J Cardiothorac Vasc Anesth 2017;31:1588-94. [Crossref] [PubMed]
- Ranucci M, Pistuddi V, Di Dedda U, et al. Platelet function after cardiac surgery and its association with severe postoperative bleeding: the PLATFORM study. Platelets 2018.1-7. [Epub ahead of print]. [Crossref] [PubMed]
- Di Dedda U, Ranucci M, Porta A, et al. The combined effects of the microcirculatory status and cardiopulmonary bypass on platelet count and function during cardiac surgery. Clin Hemorheol Microcirc 2018;70:327-37. [Crossref] [PubMed]


