
Substantial imbalance that is never eliminated with propensity score matched analyses in comparing surgery to stereotactic body radiotherapy for patients with early-stage non-small cell lung cancer
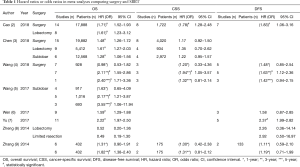
Currently, lobar resection is the standard of care for medically operable patients with early-stage non-small cell lung cancer (NSCLC), while stereotactic body radiotherapy (SBRT) is preferred for patients deemed medically inoperable and those who wish to avoid surgery (1). However, it has not yet been established and remains controversial whether surgical resection or SBRT is superior for high-risk, operable patients. Many comparative studies, including propensity score matching (PSM) analysis and meta-analyses collecting PSM data (Table 1), have been reported, with inconsistent results.
Full table
Until recently, meta-analysis data suggested that overall survival (OS) following surgery was superior to that following SBRT. However, no significant differences in cancer-specific survival (CSS) were demonstrated. Recently, Cao et al. (2) reported a systematic review and meta-analysis that included one of the greatest numbers of eligible studies and patients among all meta-analyses conducted thus far. To confirm the validity of each study, these investigators evaluated several known variables for each PSM study. Variables were divided into three categories: patient characteristics, preoperative risk factors, and tumor characteristics. The results indicated that surgery was superior to SBRT with respect to clinical outcomes, including OS and CSS, in both matched and unmatched cohorts, while SBRT was associated with fewer perioperative deaths.
Although preoperative risk factors included comorbidities, disability index, performance status, and pulmonary function tests, these cannot adequately estimate preoperative risks because these covariates were not necessarily evaluated in all the studies. Therefore, these studies failed to diminish the impact of the substantial confounding of medical operability. In addition to the meta-analysis by Cao et al., all the meta-analyses that used PSM consequently compared “medically operable patients treated with surgery” with “medically inoperable patients treated with SBRT” in a general manner. In fact, approximately 70% of matched patients treated with SBRT were deemed medically inoperable in one of the comparative studies (10). Such imbalance will not only affect OS but also CSS and disease-free survival (DFS). For example, frail patients regarded as inoperable would be less likely to receive various therapies at recurrence and/or have less physical and immunological capacity to cope with cancer, which could also affect these outcomes.
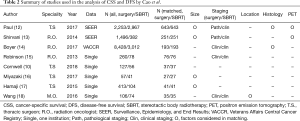
Two possible sources of substantial imbalance exist with respect to tumor characteristics. One is stage migration, i.e., staging discordance between pathological vs. clinical staging. The OS difference attributable to stage migration can be calculated using the Japanese Lung Cancer Registry data (11), which consists of prospectively collected surgical data and has substantially contributed to the International Association for the Study of Lung Cancer (IASLC) through the transfer of these data. In the survey, the 3-year OS rate in surgical patients with clinical stage IA and IB NSCLC (UICC 7th) were 89.1% and 77.6%, respectively, and those with pathological stage IA and IB disease were 92.6% and 83.4%, respectively (11). Thus, the differences in OS between clinically vs. pathologically staged patients were 3.5% and 5.8%, respectively; this represents the differences in cancer death caused by stage migration. The difference among all stage I patients is estimated to be 4.1% (3.5%×3/4 + 5.8%×1/4), assuming the ratio of stage IA to IB patients is approximately 3:1 (11). Likewise, the difference in CSS by stage migration must also be 4.1%, because the survival difference is exclusively cancer-specific. In the analysis of Cao et al. (2), approximately two-thirds of the matched surgical patients were staged pathologically and the remaining one-third were staged clinically. In contrast, all patients who underwent SBRT were staged clinically (Table 2). To estimate a potential effect of staging discordance, a hypothetical assumption is made that the treatment efficacies of surgery and SBRT are equivalent (although the staging methods are different). Thereby, the hypothetical differences in CSS between surgery and SBRT, i.e., the CSS balance due to staging discordance, is calculated to be approximately 2.7% (4.1%×2/3). In contrast, the actual 3-year rates CSS in patients treated with surgery and SBRT in the analysis of Cao et al. were 81.5% and 76.7%, respectively (Cao et al., Figure 3), with a difference of 4.8%. Therefore, the true CSS rate difference can be reduced to approximately 2.1% (range, 4.8–2.7%).
Full table
A second source of imbalance is histological subtype discordance. Most of the matched patients had pathologically confirmed disease. However, a substantial number of surgical patients would have been diagnosed only after surgery; such involved nodules are often difficult to biopsy for histological confirmation. In these patients without preoperative histological confirmation, the ratio of tumors with a major ground-glass opacity component was significantly higher than that of patients who underwent preoperative histological confirmation (19). In contrast, all patients who underwent SBRT had histologically confirmed disease before treatment, so their nodules were more likely to be composed of solid components and they tended to have a worse prognosis. Therefore, the surgical group may by default include patients with a better prognosis (20), and the difference in CSS between surgery and SBRT may be even smaller than that calculated above. Considering at least the two above imbalances, substantial biases cannot be excluded, even though the propensity analyses were technically performed well.
Surgery may actually result in better oncologic outcomes than SBRT. The extent of treatment is literally larger with lobectomy than with SBRT. In addition, mediastinal dissection (or sampling) is thought to be effective with respect to locoregional disease control. In fact, the locoregional failure rate after SBRT is 10% (21), while it is 4.9–7.7% after surgery (22).
Randomized control trials (RCT) are the only ideal way to exclude the various biases mentioned above. However, recent RCTs comparing surgery to SBRT for medically operable patients with early-stage NSCLC closed prematurely due to poor accrual. Several ongoing RCTs for medically operable or high-risk operable patients may provide important information if they are completed and have sufficient accrual. In clinical practice, more patients are integrating their personal values into treatment, such as narrative-based medicine and shared decision-making. A result from a questionnaire survey overwhelmingly favored SBRT with respect to satisfaction, impression about toxicities, and quality of life (QOL) (23). Some patients may choose a treatment strategy based on QOL rather than treatment outcome. It would be helpful to focus on the various features of both treatment strategies, such as treatment schedules, time elapsed until social reintegration, frequencies and degrees of complications, and impacts on physical, mental, and social QOL (24). In fact, the numbers of patients undergoing SBRT are gradually increasing. The American Society of Clinical Oncology (ASCO) SBRT guidelines state that, for patients with high-risk, operable stage I NSCLC, discussions about SBRT as a potential alternative to surgery are encouraged within the multidisciplinary cancer care team (25). Patients should also be invited to join the treatment decision process while awaiting the results from RCTs.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. A Takeda reports receiving a Varian research grant and a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science when conducting this study. The other authors have no other conflicts of interest to declare.
References
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Cao C, Wang D, Chung C, et al. A systematic review and meta-analysis of stereotactic body radiation therapy versus surgery for patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;157:362-73 e8.
- Chen H, Laba JM, Boldt RG, et al. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. Int J Radiat Oncol Biol Phys 2018;101:186-94. [Crossref] [PubMed]
- Wang S, Wang X, Zhou Q, et al. Stereotactic ablative radiotherapy versus lobectomy for stage I non-small cell lung cancer: A systematic review. Thorac Cancer 2018;9:337-47. [Crossref] [PubMed]
- Wang HH, Zhang CZ, Zhang BL, et al. Sublobar resection is associated with improved outcomes over radiotherapy in the management of high-risk elderly patients with Stage I non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2017;8:6033-42. [PubMed]
- Wen SW, Han L, Lv HL, et al. A Propensity-Matched Analysis of Outcomes of Patients with Clinical Stage I Non-Small Cell Lung Cancer Treated surgically or with stereotactic radiotherapy: A Meta-Analysis. J Invest Surg 2019;32:27-34. [Crossref] [PubMed]
- Yu XJ, Dai WR, Xu Y. Survival Outcome after Stereotactic Body Radiation Therapy and Surgery for Early Stage Non-Small Cell Lung Cancer: A Meta-Analysis. J Invest Surg 2017. [Epub ahead of print]. [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Zhang B, Zhu F, Ma X, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol 2014;112:250-5. [Crossref] [PubMed]
- Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. [Crossref] [PubMed]
- Sawabata N, Fujii Y, Asamura H, et al. Lung cancer in Japan: analysis of lung cancer registry cases resected in 2004. Nihon Kokyuki Gakkai Zasshi 2011;49:327-42. [PubMed]
- Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Boyer MJ, Williams CD, Harpole DH, et al. Improved Survival of Stage I Non-Small Cell Lung Cancer: A VA Central Cancer Registry Analysis. J Thorac Oncol 2017;12:1814-23. [Crossref] [PubMed]
- Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:192-201. [Crossref] [PubMed]
- Miyazaki T, Yamazaki T, Nakamura D, et al. Surgery or stereotactic body radiotherapy for elderly stage I lung cancer? A propensity score matching analysis. Surg Today 2017;47:1476-83. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Wang P, Zhang D, Guo XG, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95:e5723. [Crossref] [PubMed]
- Choi SH, Chae EJ, Shin SY, et al. Comparisons of clinical outcomes in patients with and without a preoperative tissue diagnosis in the persistent malignant-looking, ground-glass-opacity nodules. Medicine (Baltimore) 2016;95:e4359. [Crossref] [PubMed]
- Yoshiya T, Mimae T, Tsutani Y, et al. Prognostic Role of Subtype Classification in Small-Sized Pathologic N0 Invasive Lung Adenocarcinoma. Ann Thorac Surg 2016;102:1668-73. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32. [Crossref] [PubMed]
- Tong BC, Wallace S, Hartwig MG, et al. Patient Preferences in Treatment Choices for Early-Stage Lung Cancer. Ann Thorac Surg 2016;102:1837-44. [Crossref] [PubMed]
- Takeda A, Yoshida K. Decision Aid for People Facing Early Stage Lung Cancer: Choosing the treatment that is right for you. Seattle, WA: Amazon Services International, Inc., 2018.
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]


