
A novel fully covered self-expandable segmental metallic stents for the treatment of refractory esophageal stenosis
Introduction
Almost half of patients with esophageal cancer are unresectable at the time of diagnosis due to advancement or distant metastases or significant comorbidities. Those patients have a poor prognosis, with a 5-year overall survival rate of less than 15% (1,2). Complete resection and lymphadenectomy combined with preoperative chemoradiation therapy is a standard management, however, curative-intent treatment is possible in less than 40% of patients. The palliative treatment is the only option in a majority of these patients under this circumstance. The covered self-expanding metal stents (SEMS) is an effective procedure in restoring patency and relieving dysphagia (3), which has been widely used for patients with unresectable refractory esophageal fistula or esophageal stenosis (4-7). The large number of different covered metal stents available currently (8,9); however, SEMS including Choostent show “hard” connection by steel wire. The disadvantage of this stent is that it cannot bend and is not suitable for circuitous esophageal lesions. In order to overcome this shortcoming, a fully covered segmental stent by “soft” connection with nylon wire was used in this study. We aimed to study the covered self-expandable segmental metallic stents in terms of efficacy, complications, and long-term outcomes.
Methods
This study was approved by the Ethics Committee Board of our Hospital (Keyan-2015-113). All informed consents were obtained from the patients.
Study design
From March 2015 to April 2018, all patients with refractory esophageal stenosis who underwent placement of fully covered segmental stents were included and retrospectively analyzed, regardless of their histological types. The fully covered segmental stents were used in patients with refractory or circuitous esophageal stenosis confirmed by esophagography and chest computed tomography (CT) scan. Exclusion criteria included patients with mediastinal infiltration causing esophageal fistula and dysphagia due to lung cancer or lymphomas. The upper endoscopy and chest CT scan were used for the assessment of the location and length of stenosis.
Covered segmental stents
The fully covered self-expandable segmental metallic stents with double-layered polytetrafluoroethylene membrane were used in this study (ST71-224; Micro-Tech Co. Ltd, Nanjing, China). The stent was made of nitinol alloy and each stent segment was (2 cm in length) was connected with nylon wire. Both sides of stent were fully covered and both ends of the stent exceed about 5 mm. The stent had a diameter of 16, 18, or 20 mm and a length of 80, 100, 120, or 140 mm. The recovery lines were used for adjustment or removal of stent (Figure 1). The stent delivery system was 8 mm in diameter and 650 mm in length.
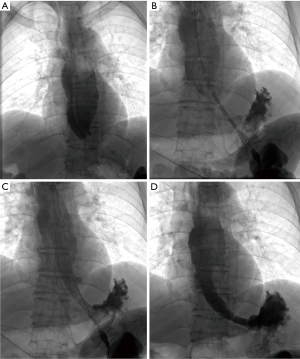
Technical details of stenting
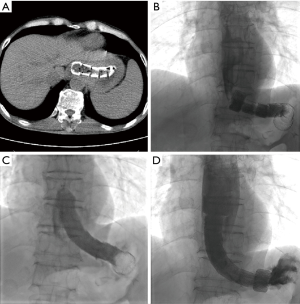
All procedure was performed under local anesthesia and fluoroscopic guidance, without the need of esophageal endoscopy. Esophagography was performed by orally take of iodine contrast agent to show the location and length of stenosis. A 5F catheter (Cook Corporation, Bloomington, USA) was introduced, and a stiff guide wire was then introduced. The covered segmental stent was implanted along the stiff guide wire. After stent placement, esophagography was performed again to confirm the patency of stent (Figure 1). A 10–14F long sheath was inserted via recovery line for stent adjustment or removal if necessary. The upper endoscopy and chest CT scan were used for the assessment of the location and length of stenosis during follow-up (Figure 2).
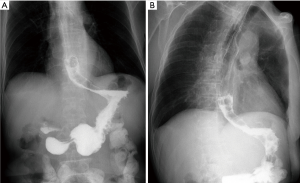
Definition
Minor complications were defined as chest discomfort or pain, insufficient stent expansion required balloon dilation, and mild gastroesophageal reflux. Major complications were defined as massive hemorrhage, stent migration or restenosis, esophageal perforation, and esophageal fistula (5). The dysphagia grade was calculated by using following scale classification: grade 0 = swallowing without dysphagia, grade 1 = dysphagia to solid food, grade 2 = dysphagia to soft solid food, grade 3 = dysphagia to liquids, and grade 4 = inability to swallow saliva or due to esophageal fistula.
Statistical analysis
Continuous variables were reported as means ± standard deviation (SD) or median with interquartile ranges (IQR) when data were not normally distributed. Categorical variables were shown as proportions. The student’s t-test and one-way ANOVA were used for comparing continuous variables by using Prism 5.0 software (GraphPad Software Inc, San Diego, USA). Significance was taken at P<0.05.
Results
Baseline demographics and indications for stenting are shown in Table 1. Twenty-four patients were enrolled in this study, 13 male and 11 female, with a median age of 74.5 years. Fifteen patients showed esophageal carcinoma, 5 patients showed carcinoma of gastric cardia and 4 patients showed compression stenosis. Thirteen patients (54.2%) showed stenosis in the lower esophagus. Three patients underwent surgical resection and 8 patients received chemotherapy or radiotherapy before stenting. A total of 24 segmental esophageal stents were used. Stent placement was technically successful in all patients. Stent removal was performed in 4 patients due to stent migration (n=3) or intolerance of stent (n=1). The median diameter and length are 18 and 110 mm, respectively. Airway stents were inserted in six patients with comorbidity of airway stenosis. Five patients underwent transarterial infusion of chemotherapy for esophageal cancer (Table 2).
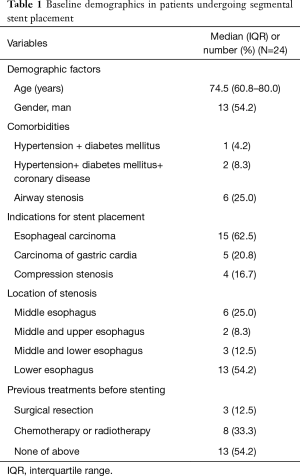
Full table

Full table
There were no perioperative deaths related to the stenting procedure. Two patients complained of mild chest pain or discomfort immediately after segmental stent placement. The pain disappeared within 3 days without any treatment. A total of 8 major complications (33.3%) were found after segmental stent placement. Stent migration was the most common complication (Figure 3), with a migration rate of 16.7% (4/24). Bleeding caused by tumor breakage was found in 1 patient about 3 hours after stenting. Complete migration was found in 3 patients, and stent removal was performed for them. Stent restenosis was found in 2 patients, with no need of treatment (Table 3).
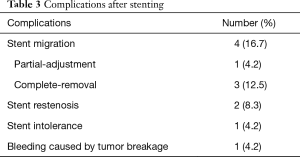
Full table
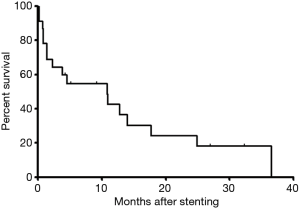
Dysphagia score decreased significantly after placement of covered segmental stent placement (P<0.0001). During a median period follow-up of 4.5 months, loss of follow-up was found in 1 patient. By the end of follow-up, 6 patients survived without obvious symptom and 17 patients were dead. The survival rates were 54.9%, 36.6%, 18.3% for 6, 12 and 24 months, respectively. The mean survival was 10.8 months (Figure 4).
Discussion
Owing to the simplicity of placement and wide spectrum of size available, SEMS have been most widely used in patients with malignant dysphagia (5,10). SEMS has proven to be technical advancement, requiring minimal dilatation, and resulting in lower morbidity and better quality of life (11,12). The large number of different covered metal stents available on the market (8,9), however, use of SEMS is not free from drawbacks or even life-threatening complications, such as the risk of migration, restenosis caused by malignant or granulomatous ingrowths, and difficulties or failure in stent removal (13,14). Besides, SEMS is often easier accepted by patients, however, no conclusive scientific evidence exists on this issue (15,16).
Commonly used SEMS show “hard” connection by steel wire, making the steel wire to form a continuous whole. Choostent is also connected by steel wire. The disadvantage of this kind of stent is that the flexibility is inadequate and it may not suitable for circuitous esophageal lesions. In order to overcome this shortcoming, a covered segmental stent by “soft” connection with nylon wire was used in the current study, which showed the advantages of flexibility in adapting to angulated stenosis. The segmental stent conforms to the esophageal curvature to the minimize the bending stress, and thus reduces the stimulation of the stent to the esophageal wall. The stent can be tightly attached to the esophageal wall in order to reduce the complication of stent migration. The stents were fully covered and both ends of the stent exceed about 5 mm, which greatly reduced the stent stimulation and minimized the incidence of restenosis. In this study, stent placement was successful in all patients with no procedure-related deaths. Stent removal was performed in 4 patients due to complications of stent migration or intolerance.
Major complications were observed in 48% and 33% of the Polyflex stent and Ultraflex stent, respectively (5). This study showed a similar rate of complication, 33.3% of major complications were found after segmental stent placement. It is reported that the migration rate ranged between 0% to 19% for Ultraflex, 6% to 17% for Polyflex stents and 4% to 9% for Flamingo stents (5,17,18). Stent migration was the most common complication in this study, with a migration rate of 16.7% (4/24), which was similar to previous reports (5,17,18). Complications requiring additional intervention are frequent (19,20). Despite the reported complications (21), we believe the covered segmental stent is still the best available palliative option in our patients.
The choice of stent diameter may represent an important factor for the successful insertion of stent. Compared to other SEMS, the use of stent with a large diameter significantly reduced the formation of granulation tissue, the chances of recurrent dysphagia, and the risk of food obstruction (22). Although Choostent placement in the oro-pharynx showed less traumatic and postprocedural pain, no significant differences in outcomes or complication rates have been reported with the available covered SEMS, such as Ultraflex, the Choostent or stent with anti-reflux valve (5,20,23). It is necessary to compare the covered segmental stent with other SEMS in the future.
In conclusion, stenting using the novel fully covered self-expandable segmental metallic stent is safe and effective in dysphagia palliation of refractory esophageal stenosis.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (grant No. 81501569).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee Board of our Hospital (Keyan-2015-113). All informed consents were obtained from the patients.
References
- Sundelöf M, Ye W, Dickman PW, et al. Improved survival in both histologic types of oesophageal cancer in Sweden. Int J Cancer 2002;99:751-4. [Crossref] [PubMed]
- Kubba AK, Krasner N. An update in the palliative management of malignant dysphagia. Eur J Surg Oncol 2000;26:116-29. [Crossref] [PubMed]
- Bethge N, Sommer A, von Kleist D, et al. A prospective trial of self-expanding metal stents in the palliation of malignant esophageal obstruction after failure of primary curative therapy. Gastrointest Endosc 1996;44:283-6. [Crossref] [PubMed]
- Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 2001;344:1681-7. [Crossref] [PubMed]
- Conio M, Repici A, Battaglia G, et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol 2007;102:2667-77. [Crossref] [PubMed]
- Bona D, Sarli D, Saino G, et al. Successful conservative management of benign gastro-bronchial fistula after intrathoracic esophagogastrostomy. Ann Thorac Surg 2007;84:1036-8. [Crossref] [PubMed]
- Włodarczyk JR, Kużdżał J. Stenting in Palliation of Unresectable Esophageal Cancer. World J Surg 2018;42:3988-96. [Crossref] [PubMed]
- Riccioni ME, Shah SK, Tringali A, et al. Endoscopic palliation of unresectable malignant oesophageal strictures with self-expanding metal stents: comparing Ultraflex and Esophacoil stents. Dig Liver Dis 2002;34:356-63. [Crossref] [PubMed]
- Nathwani RA, Kowalski T. Endoscopic stenting of esophageal cancer: the clinical impact. Curr Opin Gastroenterol 2007;23:535-8. [Crossref] [PubMed]
- Roy-Choudhury SH, Nicholson AA, Wedgwood KR, et al. Symptomatic malignant gastroesophageal anastomotic leak: management with covered metallic esophageal stents. AJR Am J Roentgenol 2001;176:161-5. [Crossref] [PubMed]
- Madhusudhan C, Saluja SS, Pal S, et al. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: impact on quality of life. Dis Esophagus 2009;22:331-6. [Crossref] [PubMed]
- Yajima K, Kanda T, Nakagawa S, et al. Self-expandable metallic stents for palliation of malignant esophageal obstruction: special reference to quality of life and survival of patients. Dis Esophagus 2004;17:71-5. [Crossref] [PubMed]
- Baron TH. A practical guide for choosing an expandable metal stent for GI malignancies: is a stent by any other name still a stent? Gastrointest Endosc 2001;54:269-72. [Crossref] [PubMed]
- Mayoral W, Fleischer D, Salcedo J, et al. Nonmalignant obstruction is a common problem with metal stents in the treatment of esophageal cancer. Gastrointest Endosc 2000;51:556-9. [Crossref] [PubMed]
- Vleggaar FP. Stent placement in esophageal cancer as a bridge to surgery. Gastrointest Endosc 2009;70:620-2. [Crossref] [PubMed]
- Lopes TL, Eloubeidi MA. A pilot study of fully covered self-expandable metal stents prior to neoadjuvant therapy for locally advanced esophageal cancer. Dis Esophagus 2010;23:309-15. [Crossref] [PubMed]
- Bethge N, Vakil N. A prospective trial of a new self-expanding plastic stent for malignant esophageal obstruction. Am J Gastroenterol 2001;96:1350-4. [Crossref] [PubMed]
- Dormann AJ, Eisendrath P, Wigginghaus B, et al. Palliation of esophageal carcinoma with a new self-expanding plastic stent. Endoscopy 2003;35:207-11. [Crossref] [PubMed]
- Ross WA, Alkassab F, Lynch PM, et al. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest Endosc 2007;65:70-6. [Crossref] [PubMed]
- Bona D, Laface L, Siboni S, et al. Self-expanding oesophageal stents: comparison of Ultraflex and Choostent. Chir Ital 2009;61:641-6. [PubMed]
- Christie NA, Buenaventura PO, Fernando HC, et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: short-term and long-term follow-up. Ann Thorac Surg 2001;71:1797-801; discussion 1801-2.
- Verschuur EM, Steyerberg EW, Kuipers EJ, et al. Effect of stent size on complications and recurrent dysphagia in patients with esophageal or gastric cardia cancer. Gastrointest Endosc 2007;65:592-601. [Crossref] [PubMed]
- Elphick DA, Smith BA, Bagshaw J, et al. Self-expanding metal stents in the palliation of malignant dysphagia: outcome analysis in 100 consecutive patients. Dis Esophagus 2005;18:93-5. [Crossref] [PubMed]


