|
Results
Bacterial RNA can form pathogen associated molecular patterns
(PAMPs) and serve as a danger signal to cells ( 10, 26). To assess
the ability of bacterial RNA to modulate PKR activation, cardiac
cells were incubated in the presence of 100 μg/mL of RNA
derived from bacterial or mammalian origins for 24 h. Total RNA
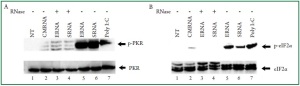
from E. coli and S. aureus were shown to be potent activators
of PKR ( Figure 1A, lane 5 and 6). Self RNA derived from
cardiac cells lacks the ability to activate PKR ( Figure 1A, lane
2). Excluding the possibility that the observed effects on PKR
activation were induced by potential contaminants in the RNA
preparations, bacterial RNA was subjected to digestion with
RNase. Digested bacterial RNA did not activate PKR suggesting
that intact bacterial RNA is required for PKR phosphorylation
( Figure 1A, lanes 3 and 4). As a positive control, poly I:C
treatment of cardiac myocytes activated PKR to an extent
comparable to that generated by bacterial RNA ( Figure 1A, lane
7 vs. lanes 5 and 6). To investigate the biological significance of PKR activation by
bacterial RNA, we tested whether bacterial RNA could induce
the phosphorylation of eIF2α. Immunolotting experiments
revealed enhanced eIF2α on Ser 51 phosphorylation induced
by bacterial RNA ( Figure 1B, lanes 5 and 6). RNase treatment
of bacterial RNA samples resulted in a reduction of the eIF2α
phosphorylation ( Figure 1B, lanes 3 and 4). In response to
bacterial RNA, the levels of eIF2α phosphorylation were
correlated to the PKR activation levels ( Figure 1A and Figure 1B,
lanes 5 and 6). To test whether the activation of PKR by bacterial RNA
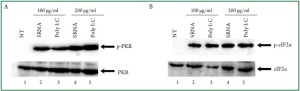
is dose-dependent, we challenged the cardiac myocytes with
bacterial RNA at 100 μg/ml or 200 μg/mL. The data from these
experiments indicated that the extent of PKR activation was
dependent on the amount of RNA that was added ( Figure 2A,
lanes 2 and 3 vs. lanes 4 and 5). However, the phosphorylation
levels of eIF2α indicated that cardiac cells treated with 200 μg/
mL bacterial RNA were not different from those treated with 100
μg/mL ( Figure 2B, lanes 2 and 3 vs. lanes 4 and 5). To further determine the role of PKR signaling in bacterial
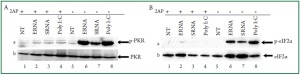
RNA recognition, we used 2-AP which is widely used as a
selective inhibitor for PKR ( 38- 40). Cardiac cells left untreated
or treated with 10 mM 2-AP for 1 h and then stimulated without
or with bacterial RNA ( Figure 3A and 3B). Bacterial RNAinduced
phosphorylation of PKR was significantly reduced when
PKR was inhibited with 2-AP ( Figure 3Aa, lanes 2 and 3 vs. lanes
6 and 7). These results suggested that recognition of bacterial
RNA is mediated by PKR. Inhibition of PKR activity by 2-AP
also resulted in a significant reduction of eIF2α phosphorylation
by bacterial RNA ( Figure 3Ba, lanes 2 and 3 vs. 6 and 7). This
observation further suggested that PKR is the kinase responsible
for induction of eIF2α phosphorylation. Although the above results demonstrated that bacterial RNA
is a potential activator for PKR, the mechanism of this activation
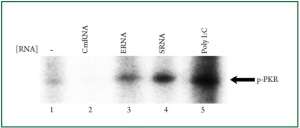
remains undetermined. Therefore, we immunoprecipitated PKR and subsequently performed in vitro PKR binding assay
to examine the ability of various RNAs to bind to the purified
PKR. The results from this assay revealed that the purified PKR
was efficiently activated by total bacterial RNA ( Figure 4, lanes
3 and 4). The efficiency of PKR activation by bacterial RNA was
comparable to that of poly I: C (Figure 4, lane 5). Cardiac RNA
failed to activate PKR ( Figure 4, lane 2). These results suggested
that bacterial RNA contains structural features that directly bind
to PKR. Therefore, PKR is a direct receptor responsible for the
recognition of bacterial RNA. Viral and bacterial RNAs share many immunostimulatory
potentials such as production of inflammatory cytokines which
is considered as a hallmark of the cellular response to nonself
RNA. These inflammatory mediators may exert pathological
effects and harm the host. It is well established that innate
immunity-mediated detection of viral dsRNA can trigger an
apoptotic response. However, there have been no reports which
describe the role of bacterial RNA as an inducer of apoptosis.
Therefore, we tested whether bacterial RNA could provoke
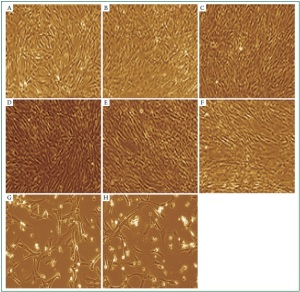
an apoptotic response. We observed that cardiac myocytes
challenged with total bacterial RNAs for 48 h exhibited a
number of morphological changes which are characteristic of
apoptosis which were cell shrinkage, membrane blebbing, and
apoptotic bodies. ( Figure 5, G and H). Digested bacterial RNA
failed to trigger these apoptotic responses ( Figure 5, B and C).
This confirms that intact bacterial RNA is the active inducer
of cardiac cell death. We examined next the involvement of
activated PKR signaling in bacterial RNA-induced cardiac
apoptosis. Resistance to apoptosis was observed when cardiac
PKR was inhibited with 2-AP ( Figure 5, E and F vs. control
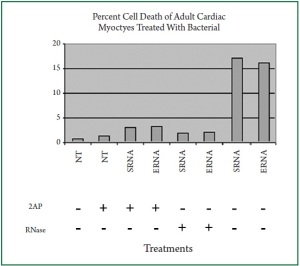
D). To assess cardiac cell viability, we performed similar
experiments using trypan blue exclusion assay. Bacterial RNA
induced an average of 17% apoptosis in the cardiac myocytes
( Figure 6). In contrast, only 2% and 3% cardiac apoptosis was
detected when the cells were treated with digested bacterial
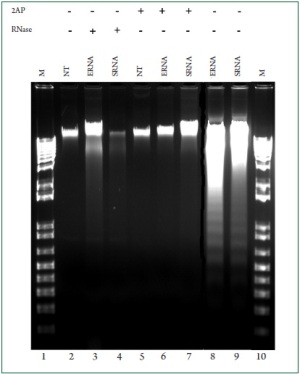
RNA and 2-AP respectively. Because DNA fragmentation is considered as a hallmark of
apoptosis, we next repeated these experiments and evaluated
the potency of bacterial RNA to trigger genomic DNA
fragmentation. Consistent with the above assays, we found that
stimulation of the cells with E. coli and S. aureus RNA induced
cardiac DNA fragmentation ( Figure 7, lanes 8 and 9). However,
genomic DNA laddering was not apparent in cardiac cells
treated with either digested bacterial RNA ( Figure 7, lanes 3
and 4) or 2-AP ( Figure 7, lanes 6 and 7). Taken together, the
above observations revealed that bacterial RNA is an inducer of
cardiac myocyte apoptosis and implicates PKR in mediating the
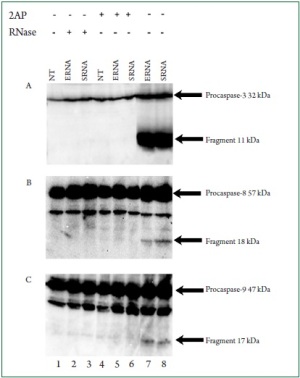
apoptotic process. To understand how bacterial RNA triggers cardiac apoptosis,
we tested the cleavage of caspases as key regulators of apoptosis.
While bacterial RNA treatment induced the cleavage of caspase 8,
caspase 9, and caspase 3 ( Figure 8 A-C, lanes 7 and 8), digested
RNA suppressed the production of active caspase fragments
( Figure 8, lanes 2 and 3). PKR inhibition by 2-AP also prevented
production of the caspases fragments (Figure 8, lanes 5 and 6).
These results suggested the requirement of PKR for bacterial
RNA-induced caspase activation.
|
|
References
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63-78.[LinkOut]
- Parrillo JE. Septic shock--vasopressin, norepinephrine, and urgency. N Engl
J Med 2008;358:954-6.[LinkOut]
- Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med
1993;328:1471-7.[LinkOut]
- Landgarten MJ, Kumar A, Parrillo JE. Cardiovascular Dysfunction in Sepsis
and Septic Shock. Curr Treat Options Cardiovasc Med 2000;2:451-459.[LinkOut]
- Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care
Nurs Clin North Am 2011;23:41-66.[LinkOut]
- Pitha PM. Unexpected similarities in cellular responses to bacterial and
viral invasion. Proc Natl Acad Sci U S A 2004;101:695-6.[LinkOut]
- Ishii KJ, Akira S. TLR ignores methylated RNA? Immunity 2005;23:111-3.[LinkOut]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate
immunity. Cell 2006;124:783-801.[LinkOut]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et
al. Species-specific recognition of single-stranded RNA via toll-like receptor
7 and 8. Science 2004;303:1526-9.[LinkOut]
- Karik?K, Buckstein M, Ni H, Weissman D. Suppression of RNA
recognition by Toll-like receptors: the impact of nucleoside modification
and the evolutionary origin of RNA. Immunity 2005;23:165-75.[LinkOut]
- Bauer S, Pigisch S, Hangel D, Kaufmann A, Hamm S. Recognition of
nucleic acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9.
Immunobiology 2008;213:315-28.[LinkOut]
- Williams BRG. The role of the dsRNA-activated kinase, PKR, in signal
transduction. Semin Virol 1995;6:191-202.[LinkOut]
- Feng GS, Chong K, Kumar A, Williams BR. Identification of doublestranded
RNA-binding domains in the interferon-induced double-stranded
RNA-activated p68 kinase. Proc Natl Acad Sci U S A 1992;89:5447-51.[LinkOut]
- Tian B, Mathews MB. Functional characterization of and cooperation
between the double-stranded RNA-binding motifs of the protein kinase
PKR. J Biol Chem 2001;276:9936-44.[LinkOut]
- Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB,
et al. Binding of double-stranded RNA to protein kinase PKR is required
for dimerization and promotes critical autophosphorylation events in the
activation loop. J Biol Chem 2001;276:24946-58.[LinkOut]
- Su Q, Wang S, Baltzis D, Qu LK, Wong AH, Koromilas AE. Tyrosine
phosphorylation acts as a molecular switch to full-scale activation of the
eIF2alpha RNA-dependent protein kinase. Proc Natl Acad Sci U S A
2006;103:63-8.[LinkOut]
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F,
et al. Autophosphorylation in the activation loop is required for full kinase
activity in vivo of human and yeast eukaryotic initiation factor 2alpha
kinases PKR and GCN2. Mol Cell Biol 1998;18:2282-97.[LinkOut]
- Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, et al. Mechanistic
link between PKR dimerization, autophosphorylation, and eIF2alpha
substrate recognition. Cell 2005;122:901-13.[LinkOut]
- Barber GN, Tomita J, Hovanessian AG, Meurs E, Katze MG. Functional
expression and characterization of the interferon-induced double-stranded
RNA activated P68 protein kinase from Escherichia coli. Biochemistry
1991;30:10356-61.[LinkOut]
- Garcí MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, et al. Impact of
protein kinase PKR in cell biology: from antiviral to antiproliferative action.
Microbiol Mol Biol Rev 2006;70:1032-60.[LinkOut]
- Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR. Double-stranded
RNA-dependent protein kinase activates transcription factor NF-kappa B
by phosphorylating I kappa B. Proc Natl Acad Sci U S A 1994;91:6288-92.[LinkOut]
- Kumar A, Yang YL, Flati V, Der S, Kadereit S, Deb A, et al. Deficient
cytokine signaling in mouse embryo fibroblasts with a targeted deletion in
the PKR gene: role of IRF-1 and NF-kappaB. EMBO J 1997;16:406-16.[LinkOut]
- Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schäer R, Kumar A, et al. Deficient
signaling in mice devoid of double-stranded RNA-dependent protein
kinase. EMBO J 1995;14:6095-106.[LinkOut]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, et al.
Double-stranded RNA-dependent protein kinase links pathogen sensing
with stress and metabolic homeostasis. Cell 2010;140:338-48.[LinkOut]
- Gil J, Alcamí J, Esteban M. Induction of apoptosis by double-stranded-
RNA-dependent protein kinase (PKR) involves the alpha subunit of
eukaryotic translation initiation factor 2 and NF-kappaB. Mol Cell Biol
1999;19:4653-63.[LinkOut]
- Koski GK, Karikó K, Xu S, Weissman D, Cohen PA, Czerniecki BJ. Cutting
edge: innate immune system discriminates between RNA containing
bacterial versus eukaryotic structural features that prime for high-level IL-
12 secretion by dendritic cells. J Immunol 2004;172:3989-93.[LinkOut]
- Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al.
RNA-associated autoantigens activate B cells by combined B cell antigen
receptor/Toll-like receptor 7 engagement. J Exp Med 2005;202:1171-7.[LinkOut]
- Bourquin C, Schmidt L, Hornung V, Wurzenberger C, Anz D, Sandholzer N,
et al. Immunostimulatory RNA oligonucleotides trigger an antigen-specific
cytotoxic T-cell and IgG2a response. Blood 2007;109:2953-60.[LinkOut]
- Hamm S, Heit A, Koffler M, Huster KM, Akira S, Busch DH, et al.
Immunostimulatory RNA is a potent inducer of antigen-specific cytotoxic
and humoral immune response in vivo. Int Immunol 2007;19:297-304.[LinkOut]
- Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi
L, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1
in response to viral infection and double-stranded RNA. J Biol Chem
2006;281:36560-8.[LinkOut]
- Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi
L, et al. Bacterial RNA and small antiviral compounds activate caspase-1
through cryopyrin/Nalp3. Nature 2006;440:233-6.[LinkOut]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, et al.
5'-Triphosphate RNA is the ligand for RIG-I. Science 2006;314:994-7.[LinkOut]
- Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R,et
al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of
antiviral immune responses. Mol Cell 2008;29:428-40.[LinkOut]
- Bevilacqua PC, George CX, Samuel CE, Cech TR. Binding of the protein
kinase PKR to RNAs with secondary structure defects: role of the tandem
A-G mismatch and noncontiguous helixes. Biochemistry 1998;37:6303-16.[LinkOut]
- Zheng X, Bevilacqua PC. Straightening of bulged RNA by the doublestranded
RNA-binding domain from the protein kinase PKR. Proc Natl
Acad Sci U S A 2000;97:14162-7.[LinkOut]
- Zheng X, Bevilacqua PC. Activation of the protein kinase PKR by short
double-stranded RNAs with single-stranded tails. RNA 2004;10:1934-45.[LinkOut]
- Kumar A, Kumar A, Michael P, Brabant D, Parissenti AM, Ramana CV, et
al. Human serum from patients with septic shock activates transcription
factors STAT1, IRF1, and NF-kappaB and induces apoptosis in human
cardiac myocytes. J Biol Chem 2005;280:42619-26.[LinkOut]
- Sugiyama T, Fujita M, Koide N, Mori I, Yoshida T, Mori H, et al.
2-aminopurine inhibits lipopolysaccharide-induced nitric oxide production
by preventing IFN-beta production.Microbiol Immunol 2004;48:957-63.[LinkOut]
- Lee JH, Park EJ, Kim OS, Kim HY, Joe EH, Jou I. Double-stranded RNAactivated
protein kinase is required for the LPS-induced activation of
STAT1 inflammatory signaling in rat brain glial cells. Glia 2005;50:66-79.[LinkOut]
- Cabanski M, Steinmüller M, Marsh LM, Surdziel E, Seeger W, Lohmeyer
J. PKR regulates TLR2/TLR4-dependent signaling in murine alveolar
macrophages. Am J Respir Cell Mol Biol 2008;38:26-31.[LinkOut]
- Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4
(TLR4) expression in cardiac myocytes in normal and failing myocardium.
J Clin Invest 1999;104:271-80.[LinkOut]
- Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor
stimulation in cardiomyoctes decreases contractility and initiates an NFkappaB
dependent inflammatory response. Cardiovasc Res 2006;72:384-
93.[LinkOut]
- Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, et al. Tolllike
receptor 2 activation by bacterial peptidoglycan-associated lipoprotein
activates cardiomyocyte inflammation and contractile dysfunction. Crit
Care Med 2007;35:886-92.[LinkOut]
- Mitchell JA, Ryffel B, Quesniaux VF, Cartwright N, Paul-Clark M. Role of
pattern-recognition receptors in cardiovascular health and disease. Biochem
Soc Trans 2007;35:1449-52.[LinkOut]
- Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in
cardiovascular disease. Nat Clin Pract Cardiovasc Med 2007;4:444-54.[LinkOut]
- Cartwright N, McMaster SK, Sorrentino R, Paul-Clark M, Sriskandan S,
Ryffel B, et al. Elucidation of toll-like receptor and adapter protein signaling
in vascular dysfunction induced by gram-positive Staphylococcus aureus or
gram-negative Escherichia coli. Shock 2007;27:40-7.[LinkOut]
- Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA
recognition by Toll-like receptors: the impact of nucleoside modification
and the evolutionary origin of RNA. Immunity 2005;23:165-75.[LinkOut]
- Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C.
Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of
uridine ribonucleotides. Eur J Immunol 2006;36:3256-67.[LinkOut]
- Hornung V, Barchet W, Schlee M, Hartmann G. RNA recognition via
TLR7 and TLR8. Handb Exp Pharmacol 2008;(183):71-86.[LinkOut]
- Bieger CD, Nierlich DP. Distribution of 5'-triphosphate termini on the
mRNA of Escherichia coli. J Bacteriol 1989;171:141-7.[LinkOut]
- Doma MK, Parker R. RNA quality control in eukar yotes. Cell
2007;131:660-8.[LinkOut]
- Maquat LE, Carmichael GG. Quality control of mRNA function. Cell
2001;104:173-6.[LinkOut]
- Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua
PC. 5'-triphosphate-dependent activation of PKR by RNAs with short
stem-loops. Science 2007;318:1455-8.[LinkOut]
- Nallagatla SR, Toroney R, Bevilacqua PC. A brilliant disguise for self RNA:
5'-end and internal modifications of primary transcripts suppress elements
of innate immunity. RNA Biol 2008;5:140-4.[LinkOut]
- Sugiyama T, Gursel M, Takeshita F, Coban C, Conover J, Kaisho T, et al.
CpG RNA: identification of novel single-stranded RNA that stimulates
human CD14+CD11c+ monocytes. J Immunol 2005;174:2273-9.[LinkOut]
- Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing
LH, Weissman D, et al. Incorporation of pseudouridine into mRNA
enhances translation by diminishing PKR activation. Nucleic Acids Res
2010;38:5884-92.[LinkOut]
- Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity
through RNA structure and the protein kinase PKR. Curr Opin Struct Biol
2011;21:119-27.[LinkOut]
- Lee SB, Esteban M. The interferon-induced double-stranded RNAactivated
protein kinase induces apoptosis. Virology 1994;199:491-6.[LinkOut]
- Kibler KV, Shors T, Perkins KB, Zeman CC, Banaszak MP, Biesterfeldt
J, et al. Double-stranded RNA is a trigger for apoptosis in vaccinia virusinfected
cells. J Virol 1997;71:1992-2003.[LinkOut]
- Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic
translation initiation factor 2 mediates apoptosis in response to activation
of the double-stranded RNA-dependent protein kinase. J Biol Chem
1998;273:2416-23.[LinkOut]
- Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNAactivated
protein kinase-dependent pathway mediating stress-induced
apoptosis. Proc Natl Acad Sci U S A 1997;94:3279-83.[LinkOut]
- Scheuner D, Patel R, Wang F, Lee K, Kumar K, Wu J, et al. Double-stranded
RNA-dependent protein kinase phosphorylation of the alpha-subunit of
eukaryotic translation initiation factor 2 mediates apoptosis. J Biol Chem
2006;281:21458-68.[LinkOut]
- Yeung MC, Liu J, Lau AS. An essential role for the interferon-inducible,
double-stranded RNA-activated protein kinase PKR in the tumor
necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci U S A
1996;93:12451-5.[LinkOut]
- Lee ES, Yoon CH, Kim YS, Bae YS. The double-strand RNA-dependent
protein kinase PKR plays a significant role in a sustained ER stress-induced
apoptosis. FEBS Lett 2007;581:4325-32.[LinkOut]
- Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW,
Archer DR, et al. Alpha/beta interferons potentiate virus-induced apoptosis
through activation of the FADD/Caspase-8 death signaling pathway. J Virol
2000;74:1513-23.[LinkOut]
- McAllister CS, Samuel CE. The RNA-activated protein kinase enhances the
induction of interferon-beta and apoptosis mediated by cytoplasmic RNA
sensors. J Biol Chem 2009;284:1644-51.[LinkOut]
- Guerra S, Lóez-Fernádez LA, Garcí MA, Zaballos A, Esteban M.
Human gene profiling in response to the active protein kinase, interferoninduced
serine/threonine protein kinase (PKR), in infected cells.
Involvement of the transcription factor ATF-3 IN PKR-induced apoptosis.
J Biol Chem 2006;281:18734-45.[LinkOut]
- Gil J, Garcia MA, Esteban M. Caspase 9 activation by the dsRNAdependent
protein kinase, PKR: molecular mechanism and relevance.
FEBS Lett 2002;529:249-55.[LinkOut]
- Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, et al. The
protein kinase PKR is required for macrophage apoptosis after activation of
Toll-like receptor 4. Nature 2004;428:341-5.[LinkOut]
- Maass DL, White J, Horton JW. IL-1beta and IL-6 act synergistically with
TNF-alpha to alter cardiac contractile function after burn trauma. Shock
2002;18:360-6.[LinkOut]
- Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res
2004;95:1140-53.[LinkOut]
- Brabant D, Michael P, Bleiblo F, Saleh M, Narain R, Tai TC, et al. Septic
sera induces apoptosis and DNA fragmentation factor 40 activation in
fibroblasts. Biochem Biophys Res Commun 2011;412:260-5.[LinkOut]
- Paladugu B, Kumar A, Parrillo JE, Der S, Osman J, Mensing J, et al. Bacterial
DNA and RNA induce rat cardiac myocyte contraction depression in vitro.
Shock 2004;21:364-9.[LinkOut]
- Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease.
Apoptosis 2009;14:536-48.[LinkOut]
- Cinel I, Ark M, Dellinger P, Karabacak T, Tamer L, Cinel L, et al.
Involvement of Rho kinase (ROCK) in sepsis-induced acute lung injury. J
Thorac Dis 2012;1:30-9.[LinkOut]
- Londoñ; D, Bai Y, Zükert WR, Gelderblom H, Cadavid D. Cardiac apoptosis
in severe relapsing fever borreliosis. Infect Immun 2005;73:7669-76.[LinkOut]
- Penninger JM, Bachmaier K. Review of microbial infections and the
immune response to cardiac antigens. J Infect Dis 2000;181:S498-504.[LinkOut]
Cite this article as: Bleiblo F, Michael P, Brabant D, Ramana CV, Tai
TC, Saleh M, Parrillo JE, Kumar A, Kumar A. Bacterial RNA induces
myocyte cellular dysfunction through the activation of PKR. J Thorac Dis
2012;4(2):114-125. doi: 10.3978/j.issn.2072-1439.2012.01.07
|