Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial
Introduction
The incidence of metastatic malignant pleural effusion (MPE) correlates with the primary tissue malignancy. Based on a recent meta-analysis, MPEs are witnessed mainly in patients with advanced lung cancer (37.5%), followed by those diagnosed with breast cancer (16.8%), lymphomas (11.5%), urogenital malignancies (9.4%) and malignancies of the gastrointestinal tract (6.9%) (1). All histological types of lung cancer are likely to exhibit metastatic MPE; adenocarcinoma is the most frequent (40%), followed by small cell lung cancer (25%) (2). The mean survival of these patients is 8 months, with 5-year survival not exceeding 2% (3). Both mean survival and 5-year survival is significantly lower compared to those with the same TNM stage, but no pleural effusion.
There is a plethora of treatment algorithms for managing patients with MPEs, sharing many common points and principles (1,4,5). The principal objective is fusion between the external surface of the pulmonary parenchyma (visceral pleura) and the inner surface of the thoracic cage (parietal pleura), effectively eliminating the pleural cavity and reaccumulation of pleural fluid. The procedure is called pleurodesis from the Greek words pleura + desis meaning “binding” (6). A popular method for inducing chemical pleurodesis is the thoracoscopic insufflation of sterile talc powder (talc poudrage) or the administration of sterile talc powder mixed with saline solution through a chest tube (talc slurry) (7). The majority of existing studies state that talc pleurodesis is a safe procedure with excellent efficacy, close to 90% (poudrage > slurry) (4,8). The underlying histological cancer type appears to affect the success. Recent studies indicate that talc pleurodesis is successful in the management of MPEs caused by large intestine, breast and ovary tumors, but the efficacy is guarded in sarcomas and gastric malignancies (6).
Hyperthermic intrathoracic chemotherapy (HITHOC), also called intrapleural perfusion thermochemotherapy (IPTC), has been used intraoperatively, following cytoreductive (debulking) surgery, when it is not possible to achieve complete (R0) tumor resection, as part of multimodality cancer treatment (9-12). In lung cancer patients with metastatic MPE, HITHOC has been applied by video assisted thoracic surgery (VATS) with good success and without major complications. The mean survival is 21.7 months, with 1-year survival of 74.1% and an improvement in performance in 89.3% of cases. Hence, HITHOC-VATS (IPTC-VATS) was introduced as a new, safe, less invasive and effective method for pleurodesis in patients with lung cancer (13). Due to its promising results in survival and quality of life, several thoracic surgeons in Europe have applied this technique with a cumulative experience of over 350 published cases (14).
The main objective of this study was to compare the two types of pleurodesis and also compare their survival as well.
Methods
Study design
This trial was conducted at a single thoracic surgery center, the “Theagenio” Cancer Institute in Greece. Approval was obtained by the Ethics committee for Human research of the Aristotle University of Thessaloniki, Greece. The whole study was mentored by three academic members from the Aristotle University of Thessaloniki and the full trial protocol was submitted in ClinicalTrials.gov under the identification code NCT01409551, with the title “Video-assisted Hyperthermic Pleural Chemoperfusion vs. Talc Pleurodesis for Malignant Pleural Effusions.”
Participants
All 40 enrolled patients were adults with cytologically proven metastatic, malignant, unilateral, pleural effusions, caused by non-small lung cancer. Exclusion criteria included patients older than 80 years, trapped lung, significantly compromised performance status (Karnofsky score <20%), congestive heart failure, recent major cardiac event (<1 year), and presence of malignant arrhythmias, bleeding diathesis, uncontrolled hypertension or diabetes mellitus, chronic renal failure, infection, previous thoracic interventions or surgery at affected side and pregnancy. Written informed consent was obtained from each study participant.
Randomization
Participants were randomly and equally assigned 1:1 to either hyperthermic intrathoracic chemotherapy (HITHOC) or talc pleurodesis (20 participants per group), following drainage of pleural effusion and cytological confirmation of disease.
Procedures
Initially, all patients had a chest tube (size 24–28 Fr) introduced to the affected hemi thorax, followed by pleural fluid drainage. After positive confirmation of diagnosis for MPE from NSCLC by the examination of three separated, consecutive pleural fluid specimens, patients randomized to HITHOC underwent VATS (two port technique) with general anesthesia, single lung ventilation and complete evacuation of pleural fluid (additional pleural tissue biopsies were performed for further histological and molecular investigations). Such was followed by continuous (45 min) intrapleural chemo-perfusion, supported by extracorporeal circulation pump (Performer HT by RanD S.A., Italy), with hyperthermic (41.5 °C), aqueous solution of carboplatin (dose: 500 mg/m2).
In participants randomized to talc pleurodesis following identical, initial steps of intra operative pleural fluid management, 8 gr of sterile talc (Steritalc by Boston Medical Products S.A., France) were insufflated in the pleural cavity. At the end of each procedure, two (HITHOC group) or one (talc group) chest tubes (28 Fr) were positioned and maintained at suction (−20 mmH2O) until the second postoperative day. Chest tubes were removed when fluid drainage was less than 150 mL per day and patients were discharged the following day. All participants received usual, standard, post-operative care, including adjuvant chemotherapy, radiotherapy and palliative care, as recommended by their attending clinicians. Follow-up was performed every month following discharge.
Outcomes
The primary outcome was the median survival from trial intervention to death.
Secondary outcomes
Secondary outcomes have been attached as a table for ease of reference and showed up their effect in the treatment outcome (Table 1). These included baseline group characteristics and also preoperative and postoperative (1st postOp, 2nd postOp and 7th postOp day) biochemistry and hematological results, including urea, creatinine, serum glutamic pyruvic transaminase (SGPT), serum glutamil oxaloacetic transaminase (SGOT), lactate dehydrogenase (LDH), C-reactive protein (CRP), sodium, potassium, calcium, albumin, white blood cells, hematocrit, hemoglobin, platelets, calculated from peripheral blood samples. Furthermore, LDH, albumin and CRP levels were measured at samples from pleural fluid preoperatively and the 7th postoperative day. Total LOS was recorded for each group.

Full table
Statistical analysis
A sample size of 40 participants was determined and patients were randomly assigned to receive either HITHOC or talc pleurodesis. The sample size was deemed sufficient enough to compensate for potential losses during the study, while being in accordance with that of other similar studies (15).
An initial baseline comparison of groups was performed evaluating general characteristics, such as demographics, biometrics, medical history, comorbidities and preoperative examination data. The analysis was further expanded to include oncological characteristics, such as primary tumor’s features, TNM staging parameters and LOS.
Quantitative variables were initially tested for normality assumption with visual inspection of histograms, box plots and normal Q-Q plots, in terms of kurtosis and skewness, as well as using formal normality tests (e.g., Shapiro Wilk test). Depending on whether normality assumption was met, either independent samples t-test or Mann-Whitney U test was run. Qualitative variables were compared between groups using a chi-square test. When expected counts in a chi-square test were lower than five, Fisher’s exact test was used instead.
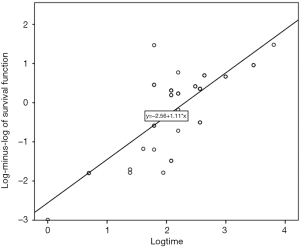
A survival analysis was performed both in the total cohort, as well as the separate treatment arms, using the Kaplan-Meier curves. Differences in survival were compared between groups, using the log-rank test, Breslow and Tarone-Ware, as per Hosmer et al. suggestions (16). Additionally, we ran a Cox proportional hazards regression analysis in order to estimate the effect of different variables on survival. A Cox regression analysis was initially performed on each variable separately. Then, a multi-factor Cox model was built up based on variables that had P values equal or lower than 0.20 and fulfilled the proportionality of hazards assumption as well as variables of clinical importance. The quality of fit in the Cox’s proportional hazards model was assessed plotting the log-minus-log (survival function) versus time log(t).
Results
Forty participants diagnosed with MPE and NSCLC were recruited in this study from August 04, 2011, to November 17, 2014. Participants were randomly and equally assigned in either HITHOC (group A) or talc pleurodesis (group B) (20 participants per arm).
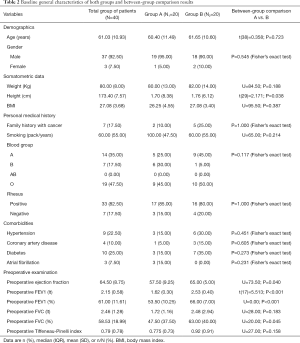
All participants were screened in order to evaluate multiple baseline group characteristics such as demographics, biometrics, medical history, comorbidities, preoperative pulmonary function tests, as well as primary tumors’ characteristics and TNM staging. Baseline between-groups comparisons have been attached as tables (Tables 2,3).
Full table

Full table
There were no deaths or major complications recorded, intraoperatively or at readmission related to procedure.
Primary end point
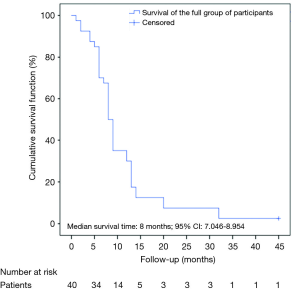
The patients were followed up for 4 years (45 months). The median survival time of the full group of participants was 8 months (95% CI: 7.046–8.954) (Figure 1).
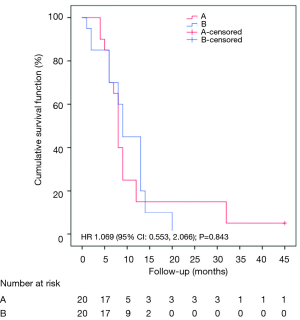
A Kaplan-Meier survival analysis was conducted to compare the two different groups. We had one censored case that belonged to group A. Participants who underwent the HITHOC had a median survival time of 8 months (95% CI: 7.141–8.859), whereas the participants of talc pleurodesis had a median survival time of 9 months (95% CI: 7.546–10.454). Following the suggestions of Hosmer et al. (16), we found that all tests, such as the log-rank, the Breslow and the Tarone-Ware tests were in concordance in that survival distributions of the two groups were not significantly different (see Figure 2) [log-rank test: χ2(1)=0.048; P=0.827; Breslow: χ2(1)=0.503; P=0.478; Tarone-Ware: χ2(1)=0.373; P=0.541]. Moreover, a Cox-regression model was conducted in order to estimate the effect of group in survival time without any significant results (HR: 1.069; 95% CI: 0.553–2.066; P=0.843).
Although the aforementioned results indicated that survival distributions of the two groups were not statistically significant, we took a cautionary approach due to survival curve crossings. As survival curves had multiple crossing points (Figure 2), the proportionality of hazards assumption was not met and therefore the log-rank test was deemed inadequate despite encouraging scientific evidence that log-rank test preserves its power (>80%) when the censored cases are few and survival curves cross at their beginning (17,18). We therefore proceeded with an alternative analysis design.
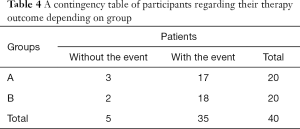
Two different analyses were planned both before and after the 14th month. As there were nil censored data before the 14th month, the chi-square test (χ2) was used to evaluate whether the therapy outcome was independent of the underlying used technique. After the 14th month we had one censored case and observed five patients who did not exhibit the event (death). In this case, survival analysis could not be implemented and descriptive data were given instead. Based on findings of χ2 test, the therapy outcome was found to be independent of the treatment group (Fisher’s exact test: P=1.000). In more detail, the proportion of patients with/without the event (death) up to the 14th month is depicted in Table 4.
Full table
In the post-14th month up to the end of the study, five participants were observed. Three of them underwent HITHOC, while two of them had talc pleurodesis. Patients of group A showed the event at the 20th month, while two of group B patients had the event at the 32th month. One censored case was mentioned in the group A.
Secondary end points
A Cox-regression model was used to estimate the effect of different factors in the treatment outcome (survival). Fifty-four factors were tested for their effects on survival, using a single-factor Cox-regression model. A multi-factor Cox model was built based on variables that had P values equal or lower than 0.20 and fulfilled the proportionality of hazards assumption, as well as variables of clinical importance (i.e., age) (Table 1).
Based on our analysis, the probability of death was 5.02 times greater in patients with MPE of stage IVB compared to that in patients with MPE of stage IVA, taking into account the effect of the other involved variables in the multi-factor model. Moreover, the quality of fit in the Cox’s proportional hazards model was assessed by plotting the log-minus-log (survival function) versus time log(t) (Figure 3). When a linear relationship is evident between these two variables, data are well-fitted in the model.
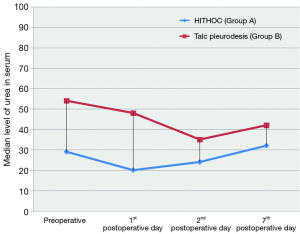
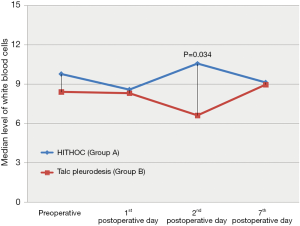
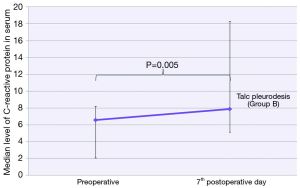
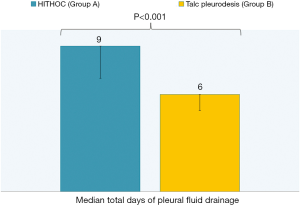
Among all recorded parameters, we would like to mention some parameters that had significant variance between the two groups. Firstly, the urea levels were significant higher in group B at all postoperative time points (1st postoperative day: U =32.00; P=0.001; 2nd postoperative day: U =46.00; P=0.004; 7th postoperative day: U =56.00; P=0.012) (Figure 4). We also observed significant elevation of levels of C-reactive protein in blood tests at 7th postoperative day only in group B (W =−2.833; P=0.005) (Figure 5). Moreover, there were significant more white blood cells (U =45.00; P=0.034) in group A at the 2nd postoperative day compared to the group B [group A: 10.55 (7.65, 13.05); group B: 6.60 (6.29, 9.11)] (Figure 6). Finally, the two groups had significant different LOS (U =7.00, P<0.001). Specifically, group A showed longer LOS compared to group B [group A: 9.00 (7.00, 9.00); group B: 6.00 (5.00, 6.00)] (Figure 7).
Discussion
Safety was confirmed in both procedures as no deaths were observed at surgery or as readmission related to procedure.
The mean survival of patients with metastatic, MPE varies among published studies. Dixit et al. reported that the mean survival of those patients was dependent on the histological type of the primary tumor and generally ranged from 3 to 12 months, and in particular for lung cancer patients it was limited to 2.6 months (19). Similarly, Clive et al. exhibited a mean survival of 2.5 months for a corresponding population (20). Zamboni et al. group recorded a median survival of 4 months in a similar population sample (21). Researchers in the Pilling et al. team reported an average survival of 7.03 months in patients with MPEs undergoing thoracoscopic chemical talc insufflation pleurodesis and 8.33 months in corresponding patients group, which underwent chemical talc slurry pleurodesis through the thoracic chest tube, bedside (22). In addition, Montero-Arias et al. reported a retrospective study of patients with MPE due to lung cancer with an average survival of 8.30 months (23). Our results showed survival comparable to the highest reported survival in the literature (8–9 months). It is also noteworthy the fact that although the hyperthermic chemoperfusion group was statistically significant different in a few of the baseline variables, such as advanced TNM stage and the preoperative respiratory tests (FEV1, FVC, FEV1/FVC), still it managed to achieve the same survival as the talc pleurodesis group. Clearly, this does not constitute a proof of one’s method superiority over the other, but it does ensure that both treatments are equally valuable.
Although there is no need in general to emphasize further that survival is dependent on TNM stage, we’d like, according to our research, to comment that TNM stage and creatinine values both preoperatively and 7 days postoperatively could be regarded as risk-factors for survival in patients with metastatic MPE. However, only TNM stage was shown to have a significant effect on patients’ survival. The same conclusion has been reached by Yoon et al., giving emphasis to the point that extrathoracic metastatic lesions exert a negative impact in patients’ prognosis (24).
Moreover, we found that groups had significant difference in length of hospital stay, with the HITHOC group A needed 2–4 more days than talc group B, due to the greater amount of postoperative fluid drainage per day. This may be considered as an indirect evidence that achieving symphysis between the visceral and the parietal pleura is faster by talc insufflation. The reported median time of hospitalization ranges from 5 to 12 days in similar groups with talc pleurodesis (25).
The fact of the significant elevation of levels of C-reactive protein in blood tests at 7th postoperative day only in talc group B has been also observed by Froudarakis et al. and attributed to inflammation caused by talc, while surgical trauma seems to play a minor role at this elevation (26). On the contrary, the significant white blood cells elevation, which revealed in HITHOC group A at 2nd postoperative day, demonstrated greater mobilization of the body’s defense systems, either as an increased response to surgical trauma or an increased response to the chemotherapeutic agent. Anevlavis et al. showed that white blood cell count >13×103/mm3 was associated with the poor survival of patients with MPE (27).
The observed urea elevated values in the group of talc pleurodesis (group B) in any time point postoperatively had been also referred by Reichner et al., while another study of Hu et al., in agreement with our study confirmed that applied intrathoracic chemoperfusion didn’t affect the renal function of patients with lung cancer (13,28). Furthermore, we concluded that creatinine levels preoperatively and in the 7th postoperative day were independent predictors significantly affecting the survival rate in both groups.
Likewise, in several international studies, ECOG performance status (Karnofsky scale) and the application of systemic chemotherapy and radiotherapy at the pre-invasive setting are very strong, independent, prognostic factors, affecting survival outcomes in patients with MPE both at single and multi-factor Cox proportional hazards models (23,24,27). Finally, recent studies indicated that the LENT score becomes the best predictor of prognostic survival for patients with metastatic, malignant, pleural effusions (22,29). These two factors were not recorded in our trial.
We did not perform any cost comparison analysis between groups, as we felt that the HITHOC procedure was more expensive than talc pleurodesis, given the cost of the perfusion consumables and chemotherapy agent compared to sterile talc. In HITHOC group, the total cost for the pump, the circuit and the technician exceeded 2,000 euros per case. Additionally, the HITHOC group exhibited longer LOS, adding to the financial difference between the two groups.
Similarly, we did not perform any debulking surgery. In our opinion, there will be always wrong calculations about the amount of tumor burden, that have been resected from each patient and each calculation will be different. Therefore, performing debulking surgery in a prospective study group, adds another undefined parameter which might affect survival.
Findings of the most recent systematic review and meta-analysis of Zhou et al. indicate that HITHOC is an effective and safe therapeutic procedure for extending patient’s life and controlling disease progress (9). We would like to note that this meta-analysis is based on MPEs which have as a causal factor any primary cancer, while our own research focuses only on non-small cell lung cancer (NSCLC).
Conclusions
The current study aimed to investigate a specific, homogeneous population and tried to highlight the efficacy of two therapeutic methods, primarily in terms of overall survival (OS), and secondarily in terms of identifying independent prognostic factors. Thorough analysis demonstrated that both intrathoracic, hyperthermic chemotherapy and talc chemical pleurodesis are equally effective and safe therapeutic options in treating patients with MPE in NSCLC with acceptable survival. There was no significant superiority between the two procedures. In the absence of survival benefit, HITHOC is more expensive and complex due to significant difference in LOS.
Despite limitations, we consider that our study has identified other parameters which showed significant changes following the application of the two different techniques and could be utilized as initiating points for new, larger clinical studies. The latter could help us draw firm conclusions in terms of understanding, monitoring and individually treating MPEs.
Acknowledgments
We highly appreciate all lab technicians for their ardent support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval was obtained by the Ethics committee for Human research of the Aristotle University of Thessaloniki, Greece (No. A6714).
References
- Roberts ME, Neville E, Berrisford RG, et al. BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Froudarakis ME. Pleural effusion in lung cancer: more questions than answers. Respiration 2012;83:367-76. [Crossref] [PubMed]
- Villena Garrido V, Cases Viedma E, Fernández Villar A, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol 2014;50:235-49. [PubMed]
- Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Malignant pleural effusion and algorithm management. J Thorac Dis 2013;5 Suppl 4:S413-9. [PubMed]
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of Pleurodesis. Respiration 2012;83:91-8. [Crossref] [PubMed]
- Colt HG, Davoudi M. The ideal pleurodesis agent: still searching after all these years. Lancet Oncol 2008;9:912-3. [Crossref] [PubMed]
- Ried M, Hofmann HS. The treatment of pleural carcinosis with malignant pleural effusion. Dtsch Arztebl Int 2013;110:313-8. [PubMed]
- Zhou H, Wu W, Tang X, et al. Effect of hyperthermic intrathoracic chemotherapy (HITHOC) on the malignant pleural effusion: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e5532. [Crossref] [PubMed]
- Hofmann HS, Wiebe K. Cytoreductive surgery and hyperthermic intrathoracic chemotherapy perfusion. Chirurg 2010;81:557-62. [Crossref] [PubMed]
- Galie N, Tabacu E. Hyperthermic intrapleural chemotherapy--another modality for the treatment of malignant pleural neoplasia. Pneumologia 2008;57:246-7. [PubMed]
- Migliore M. Debulking surgery and hyperthermic intrathoracic chemotherapy (HITHOC) for lung cancer. Chin J Cancer Res 2017;29:533-4. [Crossref] [PubMed]
- Hu R, Jiang H, Li H, et al. Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS. J Thorac Dis 2017;9:1317-21. [Crossref] [PubMed]
- Ried M, Hofmann HS, Dienemann H, et al. Implementation of Hyperthermic Intrathoracic Chemotherapy (HITHOC) in Germany. Zentralbl Chir 2018;143:301-6. [PubMed]
- Işık AF, Sanlı M, Yılmaz M, et al. Intrapleural hyperthermic perfusion chemotherapy in subjects with metastatic pleural malignancies. Respir Med 2013;107:762-7. [Crossref] [PubMed]
- Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modelling of Time-to-Event Data. Hoboken, NJ: John Wiley & Sons Inc, 2008.
- Tubert-Bitter P, Kramar A, Chalé JJ, et al. Linear rank tests for comparing survival in two groups with crossing hazards. Comput Stat Data Anal 1994;18:547-59. [Crossref]
- Li H, Han D, Hou Y, et al. Statistical Inference Methods for Two Crossing Survival Curves: A Comparison of Methods. PLoS One 2015;10:e0116774. [Crossref] [PubMed]
- Dixit R, Agarwal K, Gokhroo A, et al. Diagnosis and management options in malignant pleural effusions. Lung India 2017;34:160. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Zamboni MM, da Silva CT, Baretta R, et al. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med 2015;15:29. [Crossref] [PubMed]
- Pilling JE, Dusmet ME, Ladas G, et al. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010;5:1544-50. [Crossref] [PubMed]
- Montero-Arias F, Cartin-Ceba R, Rojas-Solano J, et al. Pleural Effusion Size as Prognostic Marker in Patients With Malignant Pleural Effusion. PLEURA 2015;2:237399751560065. [Crossref]
- Yoon DW, Cho JH, Choi YS, et al. Predictors of survival in patients who underwent video-assisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thorac Cancer 2016;7:393-8. [Crossref] [PubMed]
- Thomas R, Fysh ET, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Froudarakis ME, Klimathianaki M, Pougounias M. Systemic Inflammatory Reaction After Thoracoscopic Talc Poudrage. Chest 2006;129:356-61. [Crossref] [PubMed]
- Anevlavis S, Kouliatsis G, Sotiriou I, et al. Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration 2014;87:311-6. [Crossref] [PubMed]
- Reichner CA, Thompson JA, Kuru T, et al. Renal Insufficiency After Thoracoscopy With Talc Pleurodesis. J Bronchol 2006;13:184-7. [Crossref]
- Psallidas I, Kanellakis N, Yousuf A, et al. Lent score validation on patients with malignant pleural effusion. Eur Respir J 2016;48:PA3385.