
A prospective comparison of growth patterns with radiomorphology in 232 lung metastases—basis for patient tailored resection planning?
Introduction
Pulmonary metastasectomy is performed as a parenchyma-sparing wedge resection or enucleation with a recommended safety margin of 5–10 mm (1). Safety margins are necessary to prevent local recurrence and can be as small as 3 mm in solid organs like the liver (2). In contrast to solid organs, the lung tissue offers a wide range of possibilities for tumor cell dissemination due to its sponge-like architecture with alveolar spaces, inter-lobular septa, and it’s rich network of capillaries (3,4). Therefore it is difficult to recommend a general range of margins (5–10 mm) that accounts for any type, size and location of metastases. In a recent study, we analyzed growth pattern of pulmonary metastases and their association with local intrapulmonary recurrence. We found local intrapulmonary recurrence to be significantly correlated with aggressive growth pattern (pleural infiltration and interstitial growth), metastasis >5 mm and safety margins. Furthermore, we found a trend to fewer recurrences after resection of sharply demarcated metastases with a smooth surface. These had no tumor cell dissemination into the surrounding healthy lung tissue (5). As a consequence we hypothesize that margins can be minimized in small lesions with non-aggressive patterns of local spread. In contrast, obvious signs of local tumor spread and dissemination could warrant greater resection margins or even warrant anatomical resections. Since microscopic growth patterns can only be investigated postoperatively on the resected specimen, and are not available preoperative there is a need for growth pattern prediction on CT scans. Therefore, this study was designed to prospectively compare computed tomography (CT) morphology of metastases with the histologic patterns of local intrapulmonary spread as well as the microscopic appearance at the transition of metastasis to healthy lung tissue. The idea of growth pattern comparison with radiomorphology has already been described for lung adenocarcinoma (ADC) where margin configuration as well as solidity/ground glass opacity was associated with distinct histomorphological ADC growth patterns (6). Using the results from the comparison, we can envisage aggressive local metastasis growth related with certain patterns of radiomorphology leading to the development of individualized resection plans.
Methods
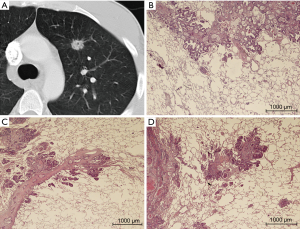
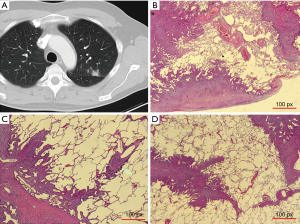
Resected pulmonary metastases were prospectively collected from patients who were operated between October 2014 and July 2016. The study was approved by the University of Essen Ethics Committee (register number 14-5914-BO). A total of 232 pulmonary metastases from 87 patients were resected in 95 operative procedures. From one to 11 lung specimens from each patient were injected and fixed with 3.5% buffered formaldehyde solution until the alveolar lung tissue had the approximate size of inflated normal lung tissue. Care was taken not to directly puncture the tumor to avoid artificial disruption of the architecture. After an incubation period of 8 hours, if present, the staple line was removed with a pair of scissors and 3 mm of safety distance was added in the specimen report. The resected area was swabbed with India ink, and the specimen was then cut into 3 mm slices beginning on the side with the thickest part of lung tissue covering the metastasis. The largest diameter of the metastasis and the shortest distance to the resection plane was noted, and up to three slices from one metastasis containing the border between the tumor and normal lung tissue were embedded in paraffin. Sections of about 5 µm were cut from each tissue block and stained with hematoxylin and eosin (H&E). All specimens were sent for regular pathologic diagnostic work-up thereafter. To establish the correct diagnosis for each patient, immunohistochemistry analysis was performed as needed. When the pathologic diagnosis was established and no further testing was necessary, all H&E slices, at least one per tumor block, were reviewed and characterized for their growth pattern at the border of the solid metastasis and the alveolar lung tissue, and zones of regression within the metastasis, such as necrosis or scarring, were noted. The classification of growth patterns was performed as described earlier (4) and included interstitial growth, satellite nodules, spread through air spaces (STAS) (Figure 1), pleural infiltration, vascular invasion (= direct invasion of vessels or spread into blood vessels, V1) or lymphatic invasion (= spread through lymph vessels, L1) as signs of aggressive local spread as well as the presence of a pseudocapsule as sign of displacing but non-infiltrating local growth. Interstitial spread, L1, V1, STAS and micro satellite nodules are further summarized as “aggressive patterns of local growth”. Additionally the surface of the metastasis was categorized into smooth (Figure 2), slightly blurred or irregular as seen with a 20-fold microscopic enlargement. The CT data of the resected nodules derived from the clinical CT scans, i.e., CT examination for staging or preoperative planning. Most scans were performed on a 64-row CT (Siemens Definition AS) using a standardized protocol (including care dose and iterative reconstruction, an 0.64 mm collimation, 1mm reconstruction interval).
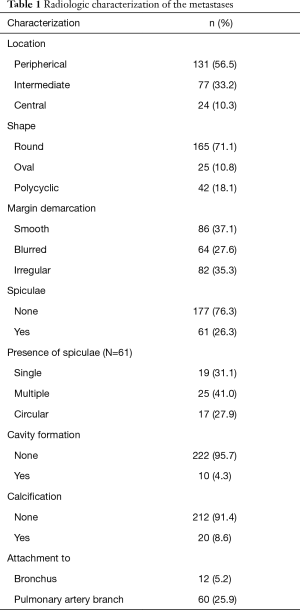
Using these CT data, the nodule corresponding to the resected specimen was systematically characterized (Tables 1,2) in the normal lung window or the 2-fold enlargement in indistinct cases. The characterization included diameter, form (round, oval, polycyclic), shape of the metastasis surface (smooth, slightly blurred, cloudy or irregular) and spiculation. The localization was defined central when the main part of the lesion was in the inner third of the lung and was defined peripheral, when the main part was in the outer third of the lung. All others were classified as intermediate. After completing the database, correlations were calculated between radiologic and histologic patterns.
Full table
Full table
Statistical analysis: The data were analyzed with the means of descriptive statistics. The evaluation of agreement between radiomorphology estimations and microscopic morphological values was performed by using Spearman’s rank-order correlation analysis considering a correlation coefficient (CC) of >0.5 as important. To determine significant differences between expected frequencies and observed frequencies in the analyzed groups the chi-squared test was used. Statistical analysis was performed using SPSS software 25.0 (SPSS Inc., Chicago, USA). A P value less than 0.05 was deemed to be statistically significant.
Results
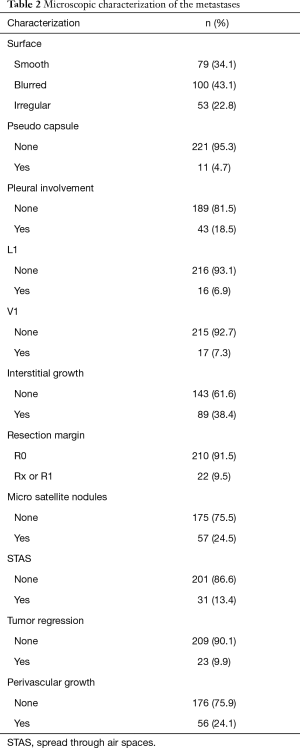
The 232 resected pulmonary metastases were microscopically categorized for the presence or absence of aggressive patterns of local growth using H&E stained slices. The baseline characteristics of patients and interventions are depicted in Table 3. Forty lesions were removed as anatomical resection (lobectomy or segmentectomy) and 192 were non-anatomical (wedge resection or enucleation) resections. VATS resection was performed in 14 patients to remove 18 lesions; all other metastases were removed by open surgery. Non-anatomical resections were performed using laser [36], electrocautery [74] or staples [82] depending on the preference of the surgeon. Pathologic R1 resection was most frequent after laser resection (16.6%), electrocautery resection (9.5%) and after using staples (1.2%) (P<0.001). There was no pathologic incomplete resection when an anatomic unit was removed. The mean metastasis diameter on CT scan (13.03 mm, SE 0.79) correlated well with the mean pathologic metastasis diameter (13.30 mm, SE 0.84) (P<0.001). The mean safety margin was 5.04 mm (median 4; 0–25 mm). Microscopic growth characteristics were found in the following frequencies: pseudo capsule 4.7%, pleural involvement 18.5%, L1 6.9%, V1 7.3%, interstitial growth 38.4%, Rx or incomplete resection (R1) 9.5%, micro satellite nodules 24.5%, STAS 13.4% (Figure 1), tumor regression 9.9%, perivascular growth 24.1% and a smooth, slightly blurred or irregular surface in 34.1%, 43.1% and 22.8%. The radiologic characteristics are depicted in detail in Tables 1,2. To demonstrate the association of radiomorphology and microscopy certain features are depicted in a crosstable (Table 4). To underline that certain features correspond well on CT scan and microscopically positive and negative statistical correlations are listed in Table 5.
Full table

Full table

Full table
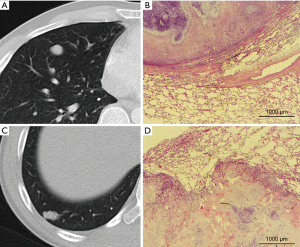
The microscopic and radiologic description of the metastasis surface correlated well (CC =0.75, P<0.001). A positive correlation of microscopic features with the surface morphology was found for interstitial growth and irregular surface (CC =0.55, P<0.001). Other sole microscopic aggressive pattern like spiculae (CC =0.142, P=0.030) and ASFC (CC =0.146, P=0.047) showed only low grade correlations with the surface radiomorphology. The radiomorphologic feature of an irregular or cloudy surface was highly associated with the presence of at least one aggressive pattern of local growth (P<0.001) (Figure 3, Table 5). A polycyclic radiologic shape was associated with at least one aggressive pattern of local growth (P=0.019) (Table 5). The presence of spiculae on CT scan was well associated with the presence of aggressive local spread (P<0.001), whereas microscopic aggressive spread was not always associated with spiculae on CT scan (P=0.149) (Table 5). Microscopic features corresponding to radiologic presence of spiculae were interstitial growth (42/61, 68.9%), L1 (10/61, 16.4%) or V1 (9/61, 14.8%). No correlation was found for the metastasis localization (central, intermediate or pheripherically) or the size with the microscopic growth patterns.
Discussion
Oncologic complete resection of tumor and preservation of lung tissue thus minimizing the postoperative functional loss are the most important opposing goals in pulmonary metastasectomy. To minimize the risk of local intrapulmonary recurrence, lobectomy with systematic lymph node dissection would be optimal to remove a lung metastasis if results from lung cancer surgery are transferred into the setting of intrapulmonary metastases. Since the overall survival benefit of metastasectomy is not proven in a prospective randomized way, there is wide spread acceptance to spare lung tissue whenever possible, especially in case of multiple lesions. On the other hand small safety margins bear the risk of local intrapulmonary recurrence, which potentially can be prevented by the surgeon with adequate resection margins. It is our understanding that personalized surgery concepts for the management of pulmonary metastasis depending on the growth pattern are helpful in preventing local recurrence. In a recent study (5) we demonstrated a correlation between some aggressive histological patterns of local spread and the frequency of local tumor recurrence. Furthermore R1 resection and small safety margins were associated with more frequent recurrences. We concluded that pulmonary metastases with aggressive local growth need larger safety margins than lesions with a smooth surface. This context offers one possibility to create a patient tailored strategy to spare lung parenchyma in cases where signs of aggressive local dissemination are absent. However, an aggressive growth pattern can only be diagnosed postoperatively in the resected specimen.
To overcome this, we conducted this prospective study comparing histomorphology with radiomorphology of pulmonary metastases. Our intention was to analyze whether aggressive patterns of local growth can be detected on preoperative CT-scans allowing a patient tailored resection based on preoperative imaging.
Encouragingly, we found a high correlation of radiologic and microscopic appearance of the metastasis surface (CC =0.75). That means that a round and smooth lesion on CT scan appears smooth and round under the microscope and an irregular appearance on CT scan usually corresponds with aggressive patterns of tumor spread under the microscope. This is an important statement as it allows to estimate the mode of metastasis local dissemination and hence the necessity of a small or large safety margin for a complete tumor resection. In further detail we found an obvious relationship between the often present smooth appearance of metastases on CT scan and smooth microscopic appearance (83.7%) equivalent to absence of aggressive local spread. Furthermore, there was no case of a smooth metastatic surface on CT scan that corresponded with an irregular appearance under the microscope. Only some cases with a microscopically slightly blurred surface could not be detected with radiology. On the other hand an irregular or cloudy surface on CT scan was highly associated with the presence of at least a feature of aggressive local spread (P<0.001) indicating a more aggressive tumor dissemination into the surrounding lung tissue. Of these, only 2.6% had a smooth surface microscopically. Then these rare cases were found clearly demarcated but had some inflammatory reaction around the lesion leading to an irregular surface on CT scan. These results support our hypothesis, that a patient tailored resection planning is possible based on radiomorphology of the lung metastases. Future studies must evaluate, if small margins for smooth lesions and larger margins for irregular lesions can be transferred into a well-balanced clinical outcome with low incidence of intrapulmonary local recurrence and a maximal preservation of lung function.
Growth patterns as prognostic factors
Other authors have demonstrated the importance of aggressive features of local spread, especially vascular invasion (V1), pleural invasion and STAS as predictor of impaired survival in colorectal cancer (CRC) lung metastases (3). STAS was identified as risk factor for local and distant tumor recurrence, and an important predictor of cancer specific survival of non-small cell lung cancer. It was found in 30% of the patients cohort and was more common with increasing tumor stage (7). Similar results were obtained in a study from Kadota et al. (8) who evaluated local recurrences after limited resection of small sized NSCLC. They found increased numbers of local recurrences in patients with STAS at the margin of the primary tumor (42.6% vs. 10.9%; P<0.001). Other authors (9) distinguished patients with aerogenous growth pattern in 29.4% (n=27), pushing pattern in 34.7% (n=32), desmoplastic pattern in 6.5% (n=6), and a mix of two growth patterns in 29.4% (n=27) after CRC lung metastasis removal. They found a poorer prognosis for patients showing an aerogenous pattern (aerogenous pattern is comparable with STAS), calculated from the time of diagnosis of the colorectal lung oligo-metastases (P=0.044). In the multivariate Cox analysis, the aerogenous pattern appeared as an independent predictor of poor overall survival (hazard ratio, 3.12; P=0.02). The authors concluded that growth patterns might be used as histology-based prognostic parameter for patients with CRC oligo metastases (9). Other authors evaluated the prognostic value of growth-pattern classification in a NSCLC database. They found papillary and alveolar growth patterns being independent predictors of early tumor recurrence (10).
Growth patterns and radiomorphology
Associations between histomorphological ADC growth patterns (lepidic, acinar, papillary, micropapillary and solid) and data from pre-operative assessment by CT were compared by Lederlin et al. (6). They found a distinct margin configuration with histomorphological ADC growth patterns. Solid-predominant ADCs usually had smooth margins and were also solid in CT scans, while lepidic-predominant ADCs had no predominant margin pattern. In addition, nonspherical tumour growth was a negative predictor of overall and disease-specific patient survival. This study also supports our hypothesis, that radiomorphology of margins influence prognosis and may be a criterion to alter standard resection regimens. Radiomics is a current subject of research. Using a panel of radiomorphologic parameters research groups demonstrated that prediction of growth patterns, mutational status and even prognosis is possible (11). As an example the amount of micropapillary component in lung ADC can be predicted from imaging parameters with clinical features. This amount has a linear correlation with prognosis and therefore image analysis can be used for treatment planning (11). Unfortunately, to the best of our knowledge, this approach has not been tested for lung metastases yet, but we see great potential for expanding radiomorphology analysis to radiomics for resection planning.
Limitations
This study has some important limitations that make simple translation into clinical use difficult. No follow-up with site focused control of local recurrence is available, since this was purely a hypothesis generating study. A validation is only possible in a prospective study where resection margins are actually adjusted to radiologic appearance. Since preoperative CT scans were in part performed in an outpatient setting involving various different radiologic departments there was a certain variability in CT section thickness. Some details at the surface of a lesion might be missed in 5 mm sections compared with 3 mm or even thin layer CT scans. Maximum intensity projection (MIP) was not routinely available. This could lead to more lesions being characterized as smooth than probably found in only 1 mm sliced CT scans. However, this minor bias does not reduce the main conclusion of this paper.
Conclusions
With this prospective comparison of imaging with pathology, we demonstrated a high concordance between radiologic and microscopic characteristics of lung metastases. A round lesion with a smooth surface detected by CT scan has an 83.7% chance to have a microscopically smooth surface and a 100% probability that it will not be microscopically irregular. In contrast the radiomorphologic feature of an irregular or cloudy surface correlated well (P<0.001) with the presence of aggressive patterns of local dissemination. This supports the hypothesis that this finding can be translated into preoperative resection planning. Smooth lesions can be marginally resected, whereas irregular or cloudy lesions need larger safety margins. The presence of spiculae indicates the presence of L1, V1 or interstitial growth.
Acknowledgments
None.
Footnote
Conflicts of Interest: Parts of the data have been presented at the National Thoracic Surgeons Congress in Germany 2017.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of the Medical Faculty of the University Duisburg-Essen register number 14-5914-BO.
References
- Rusch VW. Pulmonary metastasectomy: current indications. Chest 1995;107:322S-332S. [Crossref] [PubMed]
- Konopke R, Kersting S, Makowiec F, et al. Resection of colorectal liver metastases: is a resection margin of 3 mm enough?: a multicenter analysis of the GAST Study Group. World J Surg 2008;32:2047-56. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278-82; discussion 283. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcomas. Virchows Arch 2011;459:213-9. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Lederlin M, Puderbach M, Muley T, et al. Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 2013;41:943-51. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Yeong SJ, Pak MG, Lee HW, et al. Prognostic Utility of Histological Growth Patterns of Colorectal Lung Oligometastasis. J Pathol Transl Med 2018;52:98-104. [Crossref] [PubMed]
- Sardari Nia P, Van Marck E, Weyler J, et al. Prognostic value of a biologic classification of non-small-cell lung cancer into the growth patterns along with other clinical, pathological and immunohistochemical factors. Eur J Cardiothorac Surg 2010;38:628-36. [Crossref] [PubMed]
- Song SH, Park H, Lee G, et al. Imaging Phenotyping Using Radiomics to Predict Micropapillary Pattern within Lung Adenocarcinoma. J Thorac Oncol 2017;12:624-32. [Crossref] [PubMed]


