Implications of elevated cardiac troponin in patients presenting with acute pulmonary embolism: an observational study
Introduction
Despite the contemporary therapeutic advancement, acute pulmonary embolism (PE) remains a leading cause of hemodynamic instability and mortality (1). The important prognostic factors in patient presenting with PE include hemodynamic status and pre-existing comorbidities (2). Notably, the frequency of early deaths in patient with acute PE ranges from 7.9% to 14% (3). However, on initial presentation, it is difficult to distinguish PE from other acute cardiopulmonary disorders due to nonspecific signs and symptoms which might result in delayed or missed diagnosis and inappropriate management (4). Therefore, early diagnosis and risk-stratification of PE necessitate accurate tools which ensure timely and appropriate management (5).
Biomarkers, especially cardiac troponins (cTn) are highly sensitive and specific for myocardial injury which could be used for risk stratification in patients presented with acute PE as well to reflect the burden of PE on the right ventricle (RV) (6,7). Earlier studies have identified a correlation between cTn and degree of RV dysfunction (7,8). Furthermore, normotensive patients with RV dysfunction and elevated cTn are at increased risk of in-hospital mortality (9). A retrospective study from Qatar, reported 10% confirmed diagnosis of DVT among patients with clinical suspicion of venous thromboembolic event (VTE); of which 5% had PE (10). However, the association between cTn and clinical outcome in patients with acute PE is not well-defined yet. Therefore, the present study aims to evaluate the association between cTn status and outcome in patients with PE. It elucidated the prevalence of elevated cTns as well as the RV dysfunction in a relatively large cohort of patients with PE. We hypothesized that elevated troponins carried out a poor prognosis in PE.
Methods
It is a retrospective chart review of prospectively collected hospital based data for all consecutive patients with acute PE confirmed by Computed tomography pulmonary angiography (CTPA) between May 2011 and February 2015. All adult patients admitted for the management of acute PE at Hamad General Hospital and had at least one blood collection for cTn measurement during initial 24 h as well as RV assessment by CTPA were included in the study. The diagnosis of PE was made by CT scan examination in addition to clinical, laboratory and echocardiographic findings (10-14).
The collected data from the patients’ medical records included demographic characteristics, body mass index, presenting symptoms, co-morbidities, predisposing factors for PE, results of diagnostic procedures including coagulation profile (protein S deficiency, protein C deficiency, hyperhomocysteinemia, anti-thrombin III deficiency, and antiphospholipid syndrome), routine laboratory findings, D-dimers, cTn [i.e., cTnI or high sensitive troponin (HsTnT)] level, echocardiography (ejection fraction, RV wall hypokinesis and pulmonary arteries dilation), and CTPA findings [RV/left ventricle ratio, bowing of interventricular septum (IVS), inferior vena cava reflux, and clot burden], management (initial and long term), hospital length of stay, and mortality. An average 3 years follow-up for all-cause mortality was obtained electronically from the patients records. The severity of clinical presentation in patients with PE was assessed by clinical probability scores such as simplified Wells score, revised Geneva score and simplified pulmonary embolism severity index (sPESI). The sPESI includes 6 equally weighted variables: age >80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate ≥110 bpm, systolic blood pressure <100 mmHg, and arterial oxyhemoglobin saturation <90%. Patients with ≥1 point(s) were classified as high-risk patients, whereas low-risk patients with a score of 0 points exhibited a mortality rate of 1% (12).
As there is no well-defined definition of RV dysfunction in prior PE studies; therefore, we have used the following parameter: RV hypokinesia/dilation by echocardiography or RV/LV ratio >1.2, bowing of IVS, or IVC reflux by CTPA (14).
Based on CTPA findings, the PE was defined as the presence of an endoluminal central filling defect partially or completely occluding the pulmonary arteries (13). The present study used CTPA based scoring system proposed by Qanadli et al. (15) for quantification of the vascular obstruction index; which considered the percentage of vascular obstruction of the pulmonary arterial tree developed after PE. Briefly, the Qanadli scoring system assessed the number of blocked segmental arterial branches which were adjusted by a factor of one for partial blockage or a factor of two for complete obstruction. This score has a maximum value of 40 which corresponds to the complete occlusion of pulmonary trunk. Moreover, quantitative cardiac HsTnT and cTnI tests that detected levels higher than 14 ng/L and 0.5 ng/mL, respectively were considered as positive cTn in this study. These two tests were requested according to the discretion of attending physician. We hypothesized that PE patients with elevated cTn will have poor outcomes.
The Institutional Review Board (IRB# 15139/15) of the Hamad Medical Corporation was approved and granted exempt status for this retrospective study. The study follows the STROBE checklist for observational studies (Table S1).

Full table
Statistical analysis
Data were reported as proportion, mean (± standard deviation), median, range or IQR, when applicable. Shapiro Wilk test was performed for normality distribution of the data. Patients were categorized into two groups based on the serum cTn status (negative vs. positive) and right ventricular (RV) dysfunction (RV/LV ≤1.2 vs. RV/LV >1.2). Comparison between the two groups was done using Pearson chi-square test for categorical variables and Students t tests or Mann-Whitney test (non-normally distributed data) for continuous variables. Yates’ corrected chi-square was used for categorical variables, if the expected cell frequencies were below 5. For risk stratification, the present study attempted to combine positive cTn (hsTnT ≥0.014 ng/L or cTnI >0.5 ng/mL) as 1 plus a sPESI ≥1 point(s). The predictive value of simplified PESI, cTn result, combination of simplified PESI and cTn and clot burden was analyzed for in-hospital mortality using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+ LR), negative likelihood ratio (-LR). Area under the curve (AUC) was used to compare the discriminatory power of SI with an AUC of 1.0 considered as perfect discrimination and 0.5 considered as equal to chance. A significant difference was considered when the 2-tailed P value was less than 0.05. Kaplan Meier survival curve was constructed to display survival during the 3-year clinical follow-up with respect to troponin T status and RV dysfunction. Any differences between the curves were explored using the Log-rank, Breslow and Tarone-Ware tests. Cox regression analysis was performed after adjusting for age, sex and RV function to predict mortality in patients with and without elevated cTn; results were expressed as hazard ratio (HR) and 95% CI. Data analysis was carried out using the SPSS version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
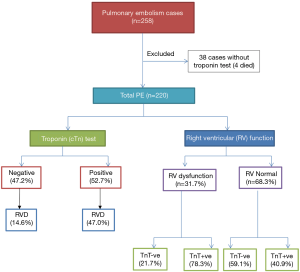
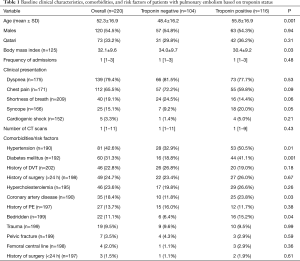
During the study period, a total of 258 consecutive patients were admitted for acute PE. Of them, cTn were not available for 38 patients and therefore they were excluded from the final analysis and 220 patients were eligible for inclusion in the study. The median follow-up was 1,097 days (1–2,065). Figure 1 shows the study design. The mean age of patients was 52.3±16.9 yrs; 120 (54.5%) were male and 100 (45.5%) were females. Positive cTn results were found in 116 (52.7%) patients and 31.7% had RV dysfunction. Table 1 shows the clinical characteristics, comorbidities, and risk factors of patients with PE based on cTn status. In comparison to the negative cTn group, patients with positive test were on average 7 years older and had lower BMI. No significant difference was observed between the 2 groups with respect to gender and clinical presentations.
Full table
The presence of hypertension (P=0.01), diabetes mellitus (P=0.001), and coronary artery disease (P=0.03) were significantly higher among patients with positive troponin than those with negative troponin results.
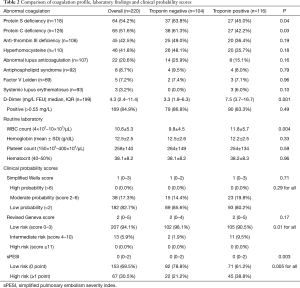
Table 2 compares the coagulation profile, laboratory findings and clinical probability scores. The deficiency of protein S (63.8% vs. 45.0%, P=0.04), and protein C (61.3% vs. 42.2%, P=0.03), were significantly higher in patients with negative troponin as compared to those with positive cTn. On the other hand, patients with positive troponin were more likely to have higher median D-dimer [7.5 (3.7–16.7)vs. 3.3 (1.9–6.3); P=0.001] than the negative cTn group.
Full table
Clinical characteristics
With respect to clinical probability scores, the majority of subjects were in low probability (82.7%) using simplified Wells score and low risk using revised Geneva score (94.1%) and simplified sPESI (69.5%). The frequency of high, moderate and low probability by simplified Wells score was comparable between the 2 groups. For revised Geneva score, patients with positive troponin were more likely to have intermediate risk of PE (9.5% vs. 1.9%; P=0.01) than the negative group. The sPESI classified 67 patients (30.5%) in the high-risk group (≥1 point). The frequency of high-risk patients by sPESI was more in the troponin positive group (P=0.005) than those who had negative cTn.
Imaging modalities
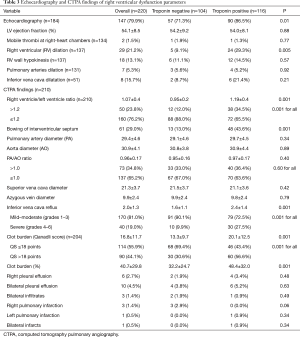
Transthoracic echocardiography findings were available in 184 cases, of which 29 patients had RV dilatation and 18 had RV hypokinesis. The left ventricular ejection fraction (LVEF), RV wall hypokinesis, pulmonary arteries dilation and inferior vena cava dilatation were comparable between the 2 groups (Table 3). The group of patients with positive troponin was more likely to have RV dilation (29.3% vs. 9.1%; P=0.005).
Full table
The parameters of the RV dysfunction by the CTPA showed that 23.8% of patients had RV/LV ratio >1.2 and 34.8% cases had higher PA/AO ratio (>1.0).
The mean clot burden (QS) was 16.8±11.7 points and 44.1% of patients had QS >18 points. In 81.0% of the patients, inferior vena cava reflux was identified to have mild-to-moderate (I–III) grades and 19.0% had severe (IV–VI) grades. Bowing of IVS was found in 61 (29.0%) patients. Higher proportion of positive troponin patients had abnormal CTPA findings in terms of higher RV/LV ratio >1.2 (34.5% vs. 12.0%; P=0.001), bowing of IVS (43.6% vs. 13.0%; P=0.001), severe grades [4–6] of inferior vena cava reflux (27.5% vs. 9.9%; P=0.001) and Qanadli score >18 points (56.6% vs. 30.6%; P=0.001).
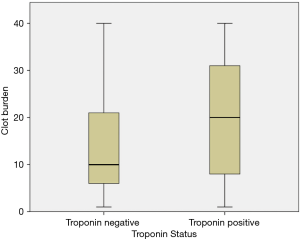
Figure 2 shows comparison of clot burden based on cTn status. The median clot burden was significantly higher in patients with positive troponin results {20 [1–40] vs. 10 [1–40]; P=0.001} as compared to those with negative troponin.
Treatment
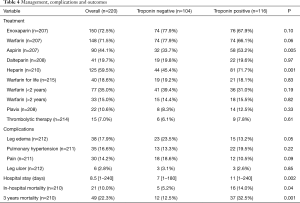
The frequently used medications were enoxaparin (72.5%), warfarin (71.5%), heparin (59.5%) and aspirin (44.1%) (Table 4). Patients with positive troponin were frequently treated with heparin (71.7% vs. 45.4%, P=0.001) and aspirin (53.2% vs. 33.7%, P=0.005) when compared to negative troponin group. Thrombolytic therapy of pulmonary clots was used in 15 patients; 9 of them had elevated troponin.
Full table
Outcomes
Patients with elevation troponin were found to have prolonged hospital length of stay as compared to those without troponin elevation. Figure 3 represents the ROC curves of HsTnT for predicting the risk of in-hospital mortality. According to the ROC curve analysis, the cutoff points of HsTnT for predicting the in-hospital mortality was 31.5 with sensitivity, 72.2%; specificity, 50.0%; and AUC 0.67 (0.52–0.81); P=0.02.
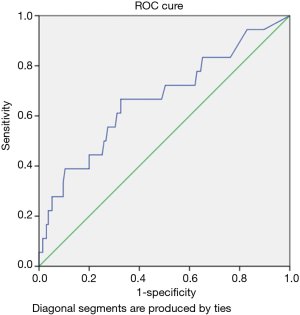
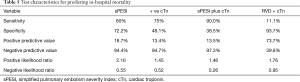
Table 5 shows the predictive value of different potential prognostic parameters with respect to in-hospital mortality. For instance, to predict the risk of in-hospital mortality; cTn test showed sensitivity 75% and NPV 94.7%, whereas the combination of sPESI with cTn troponin test showed sensitivity 90% and NPV 97.3%.
Full table
Clinico-radiological characteristics and RV dysfunction
Demographics, preexisting comorbidities/risk factors and findings of echocardiography were comparable among the two groups. However, cTn (76.0% vs. 45.0%; P=0.001), median D-dimer level [9.0 (0.2–290) vs. 3.9 (0.2–36.1); P=0.002], high-risk sPESI (54.0% vs. 25.0%; P=0.001) were significantly higher in patients with RV dysfunction. Based on radiological findings, patients with RV dysfunction were more likely to have bowing of IVS (86.0% vs. 11.3%; P=0.001), PA/AO ratio >1.0 (52.0% vs. 29.4%; P=0.003), severe inferior vena cava reflux (50.0% vs. 9.4%; P=0.001) and higher QS >18 points (84.0% vs. 31.4%; P=0.001). Also, the mean SVC diameter (22.4±3.2 vs. 20.9±3.7; P=0.01), azygos vein diameter (10.5±2.6 vs. 9.7±2.3; P=0.04) and clot burden (26.7±8.2 vs. 13.6±10.9; P=0.001) were significantly higher in patients with RV dysfunction. The mortality rate did not differ significantly among the two groups (8.0% vs. 10.1%, P=0.65).
Survival analysis
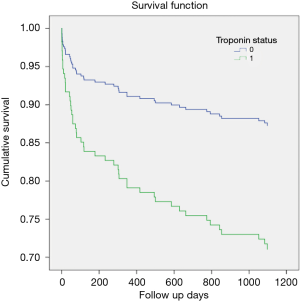
Overall mortality was 10.0% (n=21) that was significantly higher in patients with positive troponin (14% vs. 5.2%; P=0.03) in comparison to those with negative troponin. The 3-year mortality was 22.3% (+ve cTn 32.5% vs. –ve cTn12.5%, P=0.001).
Figure 4 shows the Kaplan-Meier survival curves with significantly higher mortality in the positive troponin group than negative group (Log-rank: X2=11.3, P=0.001, Breslow: X2=11.0, P=0.001 and Tarone-Ware: X2=11.2, P=0.001). However, no significant difference was observed for mortality with respect to RV dysfunction Log-rank: X2=0.45, P=0.49, Breslow: X2=0.29, P=0.58 and Tarone-Ware: X2=0.37, P=0.54).
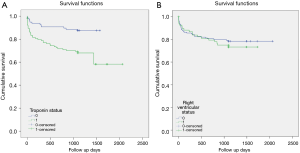
Cox regression analysis showed that mortality increased 3 times with positive cTn after adjusting for age and RV dysfunction (adjusted HR 2.5; 95% CI: 1.25–5.01), P=0.009) (Figure 5).
Discussion
This is a large cohort study that analyzes the association between elevated cTn and acute PE from an Arab Middle Eastern country. Interestingly, a number of studies have identified an association of elevated cTn levels in patients with acute PE with an incidence of 30% to 40% (6,16,17). In this study, more than a half of PE patients had elevated cTn and such patients were more likely to be elderly with more comorbidities. Cox regression analysis showed that regardless of the age, sex and RV dysfunction, an elevated cTn is associated with 2.5-fold increase at the risk of long-term mortality. Consistent with our findings, a study from Austria reported an association between advanced age, higher comorbidities, presentation with severe clinical manifestations of PE and elevated cTn (11). The possible explanation for the release of cTn is attributed to acute RV strain, decreased coronary blood flow, and hypoxemia secondary to PE (18). The RV is integral to normal cardiac function; however, the crescent-shaped geometry of the RV makes the chamber poorly tolerant of acute elevations in pulmonary circulation which can cause acute RV dilation, damaging the contractile sarcomere apparatus (19) with resultant RV dysfunction and troponin and BNP release.
cTn has a prognostic relevance for risk stratification and outcome of PE due to its high negative predictive value (11,12). Moreover, in patients with acute PE, increased levels of cTn indicate a significant RV strain which might be related to the higher risk of severe PE as well as a higher overall mortality (6,20,21). Therefore, detection of negative troponin might be useful for identifying low-risk patients that could be managed without even hospitalization (22). In the current study, elevated cTn showed a higher association with sPESI scores, RV dilation on echocardiography, CTPA parameters, prolonged hospital course and higher rate of mortality. Early assessment of the severity for acute PE is crucial for accurate decision making and initiation of the first line treatment (23). Among various prognostic scoring, sPESI was considered as the most useful and validated tool to predict mortality in patients with PE (24). In the present study, based on sPESI score, one-third of patients were identified to have high-risk. Importantly, hemodynamically unstable patients who were considered as high-risk patients, were found to have more than 15% chance of early death; while, patients categorized as low-risk showed a better prognosis (25). However, the calculation of PESI is complex involving 11 different parameters. In our study, high risk sPESI was more in patients with positive cTn in comparison to the negative cTn group (38.8% vs. 21.2%).
In our study, deficiencies of protein S, protein C and anti-thrombin III were the most common hereditary risk factors of PE which is consistent with an earlier study (26). Earlier reports have demonstrated the utility of D-dimer testing for risk assessment in patients with acute PE (27,28). There was a relationship between elevated D-dimer levels with the severity of acute PE, RV dysfunction, clot burden, and mortality in acute PE cases (29). In the current study, median D-dimer values in the troponin positive group were found to be higher when compared to negative cTn group.
Furthermore, some investigators found a significant relationship between NT-proBNP, troponin levels, and RV dysfunction in PE and recommended their measurement at the earliest for better risk stratification (30). Several studies have demonstrated a significant correlation between RV dysfunction on echocardiography and elevated troponin levels (31,32). Notably, echocardiographic RV dysfunction was found to be an independent predictor of mortality in patients with acute PE, regardless of the hemodynamic status (30).
In our series, we have observed a significant correlation between cTn status and clot burden which is in agreement with an earlier study (32). Studies using CTPA findings to diagnose RV dysfunction were more reliable and many of them have assessed its association with troponin levels (33). In the present study, 24% of the patients had a RV/LV ratio of >1.2 which was also significantly higher in patients with positive troponin as described by Jeebun et al. (32). The authors reported that in patients with elevated troponin levels, 90% had a RV/LV ratio of >0.9 which is higher as compared to our study (34.5%) which is attributed to the lower RV/LV ratio (>0.9) used in Jeebun study.
The present study also observed a significant association between positive cTn and CTPA findings. Similar to our findings, an earlier report identified an association of leftward bowing of IVS with elevated cTn levels (32). The present study re-confirms findings of our previous finding that patients with RV dysfunction (RV/LV ratio >1.2) were more likely to have greater clot burden, and septal bowing (34).
Arram et al. (35) suggested that troponin and myoglobin testing can be integrated with risk assessment of acute PE to guide treatment. The authors concluded that PE patients with normal troponin and myoglobin values could be successfully managed with anticoagulation therapy alone.
The overall in-hospital mortality in our cohort was 10.0% and the 3-year mortality was 22.3%. Patients with positive troponin were found to have two to three-fold increased risk of early or long-term mortality. Similar to our findings, Giannitsis et al. (18) reported a higher rate of in-hospital mortality in patients with positive cTnT (44%) compared to those with negative cTnT (3%). Another study by Pruszczyk et al. (36) included 64 normotensive patients with acute PE. The authors reported that all eight patients who died in the hospital had elevated troponin; whereas, all the survivors had no signs of myocardial injury. A recent meta-analysis included 22 studies of low-risk PE patients to assess the prognostic implications of RV dysfunction and elevated cardiac biomarkers (37). The authors concluded that RV dysfunction on initial presentation is associated with early mortality among low-risk PE patients. Also, imaging based diagnosis of RV dysfunction seems to have similar prognostic implications as that of the elevation of cardiac biomarkers. We noticed that the sensitivity, NPV and -LR have been strengthened significantly when we combined troponin status with the sPESI for prediction of mortality (Table 5). Similarly, Singanayaga et al. (23) suggested that addition of cTn to PESI score have better predictive value for 30-day mortality in patients with acute PE. Therefore, it can be used to guide initial management of normotensive PE patients.
Timely identification of imaging based RV dysfunction can provide indirect clue of imminent hemodynamic failure in normotensive PE patients which may lead to PE-related shock (25). Earlier investigators considered RV dysfunction in PE based on RV/LV ratio (ranges 1 to 1.5) and degree of obstruction which also has potential to predict of PE-related mortality (37,38). Moreover, the assessment of circulatory collapse and severity of PE could be more precisely made by RV dysfunction rather than the clot burden (39). In the present study, patients with RV dysfunction were more likely to have elevated cTn, D-dimer level, and had high-risk sPESI. Consistent with our findings, an earlier study revealed that two-third of the PE patients had elevated cTnI which was significantly associated with RV dysfunction (34). Similarly, Rydman et al. (40) reported that patients with high-risk sPESI had higher association with RV dysfunction.
Limitations
The present study has certain limitations owing to the retrospective design which might influence the generalizability of the findings. Selection bias cannot be ruled out as all patients with PE who underwent CTPA and cTn testing were included in the analysis. We missed cTn measurement in 14.7% of cases, of them 4 cases died. Also echocardiography was missed in 16% of cases whereas all cases underwent CTPA scanning.
Another limitation is that we did not consider the predictive value of other cardiac biomarkers such as BNP, which had association with adverse clinical outcomes in earlier studies (37).
In addition, 2 assays of troponin were used; however, the timing and indication, and the choice between 2 assays were not explained. Myoglobin and BNP were not measured in our cohort. The exact cause of death during the 3 years follow-up was not reported.
Conclusions
Measurement of cTn is a useful additional prognostic indicator for risk stratification and long term outcome in patients with acute PE event. cTn, if combined with clinical probability score has higher predictive power to identify PE patients at increased risk of mortality. Moreover, radiological findings of RV dysfunction also have clinical implications in PE patients. Therefore, the association between cTn and RV dysfunction indicates a link between acute elevation of RV pressure and severity of PE. Furthermore, larger prospective studies are needed to confirm the findings of the present study. Also, we need to know whether the clinicians have realized the importance of measuring cTn in PE and changed their routine management approach based on the cTn status.
Acknowledgments
We would like to thank all the staff of Vascular and Radiology Department at Hamad General Hospital, for their contribution and support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been performed in accordance with the ethical standards. The study obtained ethical approval from Research Ethics Committee, at Medical Research Center, Hamad Medical Corporation (HMC), Doha, Qatar (IRB#15139/15). A waiver of consent was granted as no direct contact with patients and data were anonymous.
References
- Meyer T, Binder L, Hruska N, et al. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol 2000;36:1632-6. [Crossref] [PubMed]
- Ozsu S, Abul Y, Orem A, et al. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip Respir Med 2013;8:34. [Crossref] [PubMed]
- Tanabe Y, Obayashi T, Yamamoto T, et al. Predictive value of biomarkers for the prognosis of acute pulmonary embolism in Japanese patients: Results of the Tokyo CCU Network registry. J Cardiol 2015;66:460-5. [Crossref] [PubMed]
- Ji QY, Wang MF, Su CM, et al. Clinical symptoms and related risk factors in pulmonary embolism patients and cluster analysis based on these symptoms. Scientific Reports 2017;7:14887. [Crossref] [PubMed]
- Hogg K, Haslam S, Hinchliffe E, et al. Does high- sensitivity troponin measurement aid in the diagnosis of pulmonary embolism? J Thromb Haemost 2011;9:410-2. [Crossref] [PubMed]
- Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007;116:427-33. [Crossref] [PubMed]
- Demir N, Ekim N, Oguzulgen IK, et al. The Value of Cardiac Troponins in Diagnosis and Differential Diagnosis of Pulmonary Embolism. J Pulmon Resp Med 2012;2:8.
- Müller-Bardorff M, Weidtmann B, Giannitsis E, et al. Release Kinetics of Cardiac Troponin T in Survivors of Confirmed Severe Pulmonary Embolism. Clin Chem 2002;48:673-75. [PubMed]
- Jiménez D, Uresandi F, Otero R, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest 2009;136:974-82. [Crossref] [PubMed]
- Al-Thani H, El-Menyar A, Asim M, et al. Clinical Presentation, Management, and Outcomes of Deep Vein Thrombosis Based on Doppler Ultrasonography Examination. Angiology 2016;67:587-95. [Crossref] [PubMed]
- Janata KM, Leitner JM, Holzer-Richling N, et al. Troponin T predicts in-hospital and 1-year mortality in patients with pulmonary embolism. Eur Respir J 2009;34:1357-63. [Crossref] [PubMed]
- Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary EmbolismSeverity Index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011;124:2716-24. [Crossref] [PubMed]
- Furlan A, Aghayev A, Chang CC, et al. Short-term mortality in acute pulmonary embolism: Clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology 2012;265:283-93. [Crossref] [PubMed]
- Praveen Kumar BS, Rajasekhar D, Vanajakshamma V. Study of clinical, radiological and echocardiographic features and correlation of Qanadli CT index with RV dysfunction and outcomes in pulmonary embolism. Indian Heart J 2014;66:629-34. [Crossref] [PubMed]
- Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: Comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176:1415-20. [Crossref] [PubMed]
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003;108:2191-4. [Crossref] [PubMed]
- Thielmann M, Pasa S, Wendt D, et al. Prognostic significance of cardiac troponin I on admission for surgical treatment of acute pulmonary embolism: a single-centre experience over more than 10 years. Eur J Cardiothorac Surg 2012;42:951-7. [Crossref] [PubMed]
- Giannitsis E, Müller-Bardorff M, Kurowski V, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000;102:211-7. [Crossref] [PubMed]
- Matthews JC, McLaughlin V. Acute Right Ventricular Failure in the Setting of Acute Pulmonary Embolism or Chronic Pulmonary Hypertension: A Detailed Review of the Pathophysiology, Diagnosis, and Management. Curr Cardiol Rev 2008;4:49-59. [Crossref] [PubMed]
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425-30. [Crossref] [PubMed]
- Douketis JD, Leeuwenkamp O, Grobara P, et al. The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism. J Thromb Haemost 2005;3:508-13. [Crossref] [PubMed]
- Moores L, Aujesky D, Jiménez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost 2010;8:517-22. [Crossref] [PubMed]
- Singanayagam A, Scally C, Al-Khairalla MZ, et al. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM 2011;104:125-31. [Crossref] [PubMed]
- Shafiq A, Lodhi H, Ahmed Z, et al. Is the Pulmonary Embolism Severity Index Being Routinely Used in Clinical Practice? Thrombosis 2015;2015:175357. [Crossref] [PubMed]
- Kang DK, Sun JS, Park KJ, et al. Usefulness of combined assessment with computed tomographic signs of right ventricular dysfunction and cardiac troponin T for risk stratification of acute pulmonary embolism. Am J Cardiol 2011;108:133-40. [Crossref] [PubMed]
- Goldhaber SZ. Risk factors of venous thromboembolism. J Am Coll Cardiol 2010;56:1-7. [Crossref] [PubMed]
- Becattini C, Lignani A, Masotti L, et al. D-dimer for risk stratification in patients with acute pulmonary embolism. J Thromb Thrombolysis 2012;33:48-57. [Crossref] [PubMed]
- Aujesky D, Roy PM, Guy M, et al. Prognostic value of D-dimer in patients with pulmonary embolism. Thromb Haemost 2006;96:478-82. [Crossref] [PubMed]
- Yazıcı S, Kırış T, Ceylan US, et al. The accuracy of combined use of troponin and red cell distribution width in predicting mortality of patients with acute pulmonary embolism. Wien Klin Wochenschr 2016;128:596-603. [Crossref] [PubMed]
- Choi HS, Kim KH, Yoon HJ, et al. Usefulness of cardiac biomarkers in the prediction of right ventricular dysfunction before echocardiography in acute pulmonary embolism. J Cardiol 2012;60:508-13. [Crossref] [PubMed]
- Aksay E, Yanturali S, Kiyan S. Can elevated troponin I levels predict complicated clinical course and inhospital mortality in patients with acute pulmonary embolism? Am J Emerg Med 2007;25:138-43. [Crossref] [PubMed]
- Jeebun V, Doe SJ, Singh L, et al. Are clinical parameters and biomarkers predictive of severity of acute pulmonary emboli on CTPA? QJM 2010;103:91-7. [Crossref] [PubMed]
- Hsu JT, Chu CM, Chang ST, et al. Prognostic role of right ventricular dilatation and troponin I elevation in acute pulmonary embolism. Int Heart J 2006;47:775-81. [Crossref] [PubMed]
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006;25:181-6. [PubMed]
- Arram EO, Fathy A, Abdelsamad AA, et al. Value of cardiac biomarkers in patients with acute pulmonary embolism. Egypt J Chest Dis Tuberculosis 2014;63:247-52. [Crossref]
- Pruszczyk P, Bochowicz A, Torbicki A, et al. Cardiac troponin T monitoring identifies high-risk group of normotensive patients with acute pulmonary embolism. Chest 2003;123:1947-52. [Crossref] [PubMed]
- Barco S, Mahmoudpour SH, Planquette B, et al. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2019;40:902-10. [Crossref] [PubMed]
- El-Menyar A, Nabir S, Ahmed N, et al. Diagnostic implications of computed tomography pulmonary angiography in patients with pulmonary embolism. Ann Thorac Med 2016;11:269-76. [Crossref] [PubMed]
- Miller RL, Das S, Anandarangam T, et al. Association between right ventricular function and perfusion abnormalities in hemodynamically stable patients with acute pulmonary embolism. Chest 1998;113:665-70. [Crossref] [PubMed]
- Rydman R, Söderberg M, Larsen F, et al. d-Dimer and simplified pulmonary embolism severity index in relation to right ventricular function. Am J Emerg Med 2013;31:482-6. [Crossref] [PubMed]