
Poor enhancement pattern of left atrial appendage in cardiac computed tomography is associated with stroke in persistent atrial fibrillation patients
Introduction
Atrial fibrillation (AF) is one of the most prevalent arrhythmias and most common causes of cardiogenic thromboembolism including stroke/transient ischemic attack (TIA) (1-4).
In AF patients, left atrial appendage (LAA) is the major source of thrombi causing stroke/TIA. To prevent thrombi from developing in the LAA, anticoagulation therapy is initiated in thromboembolism high-risk AF patients (5,6). The CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA to thromboembolism, vascular disease, age 65–74 years, and sex category) is an effective evaluation method relevant to the risk of stroke and widely used for clinical decision-making regarding whether or not anticoagulation therapy should be initiated (7). While the CHA2DS2-VASc score is practical and useful, evaluating the LAA morphologically and hydrodynamically can also be useful, representing another aspect of the CHA2DS2-VASc score. Occlusion of the LAA as the source of thrombi has recently been highlighted as an effective approach for preventing stroke in AF patients (8-11). Although the LAA is involved in thrombus formation in AF patients, a practical method for evaluating the state of the LAA has yet to be developed.
In previous studies, many factors of the LAA are investigated: the morphology, size, volume, orifice area, and velocity to the LAA (12-16). A number of techniques for evaluating the LAA have also been examined, and transesophageal echocardiography is traditionally used to clarify the kinetic hemodynamics (17-20), with computed tomography (CT) has been recently used to clarify the static hemodynamics (20-22). Defects in medium contrast in the LAA have been detected in some AF patients and was regarded as an indicator of LAA thrombus (20,23,24).
In the present study, we assessed the hypothesis that contrast medium defects seen only in the early phase with none in the late phase demonstrated congestion of the blood flow to the LAA but not thrombus, thereby creating a high risk of thromboembolism. We studied the medium contrast enhancement patterns in the LAA by CT to identify different LAA blood flow patterns and tried to correlate the different patterns with the patient history of cardiogenic stroke.
Methods
Patients
The patient population in this study consisted of 147 consecutive patients with chronic AF planning to undergo cardiac surgery or catheter ablation in two institutions (Tsukuba Memorial Hospital, Tsukuba, Japan). In preparation for these procedures, CT was performed not explicitly for this study. The CHADS2 and CHA2DS2-VASc score of each patient was evaluated when the CT was performed, so the surgery or ablation had no influence on this study. In addition, only the factor of stroke/TIA history was evaluated before the last occurrence of stroke/TIA history. If recurrent stroke happened, then two points were added for a “history of stroke”. It is because the factor whether the history of stroke/TIA exists is the criteria of dividing the patients into the two groups of “No stroke” and “Prior stroke”. This method is previously reported in the paper about LAA evaluation (12). The LAA enhancement pattern on CT was then classified into three different categories of risk (as discussed later).
CT
Contrast-enhanced cardiac CT was performed with a 64-slice CT scanner (Aquilion CXL, Toshiba Medical Systems, Tochigi, Japan) with a standardized protocol, as previously reported (23,25). The same protocol was used for both patients with AF and those with sinus rhythm.
After a preliminary planning scan, the deviation of the heart rate during breath-holding was checked and calculated using a HeartNAVI application (Toshiba Medical Systems). Based on the evaluation of the heart rate, the helical pitch and the delay time between breath-holding and the scan starting was determined.
A bolus of contrast media with 370 mgI/mL iopamidol was injected intravenously. The dose was decided based on the patient’s body weight, and the injection rate was modified to allow for an injection time of 17 seconds.
After the CT attenuation value in the ascending aorta reached 150 HU, the breath-holding information was obtained automatically. After the predefined delay time, the helical scan started. The whole volume of the heart was scanned during a single breath-hold of approximately 10 seconds in a typical case. No specific adjustment protocol for the LAA or AF was implemented. The detector collimation was 64×0.5 mm, and the tube voltage was 120 kVp. Gantry rotation was adjusted for the helical pitch speed, which usually ranged from 350–400 ms/rotation. The raw images were reconstructed to generate 1-mm-thick slices. All images were reviewed by two cardiologists and one radiologist independently. All of the reviewers were blinded to the history of stroke. The final decision regarding the risk pattern was made by consensus. The inter-observer variability was small, with detailed data reported in the Results.
Classification of the LAA enhancement pattern
Based on the extent of enhancement in the LAA in early phase, three enhancement patterns were established:
Poor enhancement pattern
A clear and linear boundary between the well-enhanced area and unfilled area (Figure 1). Checking the contrast medium status in the LAA in the late phase as well was important, as this confirmed that the filling defect in the early phase was not a thrombus. This method of LAA thrombus exclusion was previously reported (25). This pattern was also called a “pseudo-defect”.
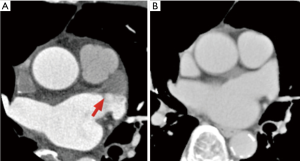
Good enhancement pattern
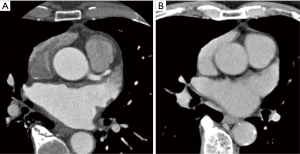
Contrast medium fully fills the LAA in the early phase (Figure 2). The enhancement pattern of the LAA was the same as the pattern in the late phase.
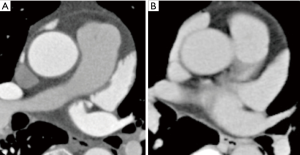
Intermediate enhancement pattern
All enhancement patterns other than the poor and good enhancement patterns (Figure 3). We classified patterns that could not be confirmed as good or poor enhancement patterns into this category, so this category includes substantial variation.
Statistical analysis
Continuous data are presented as the mean ± standard deviation and were compared using the t-test for normally distributed data and Kruskal-Wallis test for three LAA enhancement patterns. Categorical variables are presented as the count and percent and were compared using Fisher’s exact test. CT images were blindly and independently evaluated by one cardiologist and one radiologist, who categorized the LAA enhancement patterns into the three categories described above. The interobserver reliability among the evaluators was determined by Cohen’s Kappa; a kappa value >0.61 was regarded as indicating a good level of agreement (26).
The classification was finally determined by the evaluation which the 2 of 3 readers judged when the three evaluations were not same. In no case, all the three readers judged different classifications.
A multivariate logistic model was used to verify new indicators as significant independent risk factors of stroke. All potential factors were entered into the logistic model, based on the previously known clinical indicators (e.g., CHA2DS2-VASc score). The CHA2DS2-VASc excluding the factor of stroke/TIA history score was calculated when the CT was performed, and 2 points were added to the history of stroke/TIA only when it was recurrent. This reason was discussed above.
In all tests, a P value <0.05 was considered statistically significant.
Statistical analyses were performed with the EZR software program (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R foundation of Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics (27).
Ethics
This study obtained ethics approval of institutional review board (Tsukuba Memorial Hospital Ethics Committee, approval number H-27901), and the participants gave informed consent before taking part.
Results
The CT images and clinical records of 147 consecutive patients with AF who underwent cardiac CT were collected. The patients’ characteristics was as follows: age 69±9 years, 82% male, ejection fraction 57%±12%, and 42% with CHA2DS2-VASc score >1. Warfarin was given to 59 patients, so was dabigatran to 24 patients, rivaroxaban to 31 patients, and apixaban to 27 patients. Six patients did not take coagulants due to some reasons; side effect of bleeding, cost, or allergy. The prevalence of poor, intermediate, and good enhancement patterns of the LAA was 72 (49%), 33 (22%), and 42 (29%), respectively.
No statistically significant bias was detected in categorizing the LAA enhancement patterns by operators (cardiologist and radiologist), and the kappa values are as follows: kappa =0.80, 95% confidence interval (CI): 0.63–0.97, P=0.01.
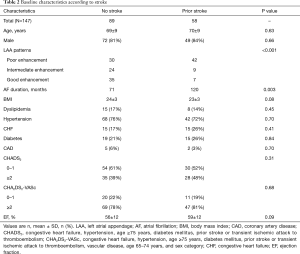
Table 1 presents the baseline characteristics of the whole patients and the populations sorted by three LAA patterns. No marked differences between the groups were found in the prevalence of dyslipidemia, coronary artery disease, or diabetes mellitus, or in the ejection fraction. However, the groups did differ in the AF duration, hypertension, congestive heart failure, history of stroke, and CHADS2 score. In all cases, the heart rate was controlled under 75 beats per minute when cardiac CT performed.
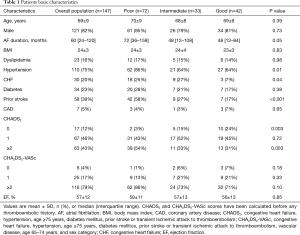
Full table
Prevalence of stroke
In this study, 58 (39%) patients had a history of stroke. The distribution of stroke history differed among the LAA patterns: 42 (58%), 9 (27%), and 7 (17%) for poor, intermediate, and good enhancement patterns, respectively (P<0.001) (Table 1). In particular, a history of a stroke history was significantly more prevalent in those with a poor enhancement pattern (58% vs. 21%, P<0.001) that in those with other patterns combined with good and intermediate patterns. Table 2 shows the clinical characteristics of the patients with and without a previous stroke history. Those with a poor enhancement pattern of LAA had a significantly higher rate of stroke than those with other patterns. The CHA2DS2-VASc score was not a significant factor between the two groups.
Full table
Univariate analysis
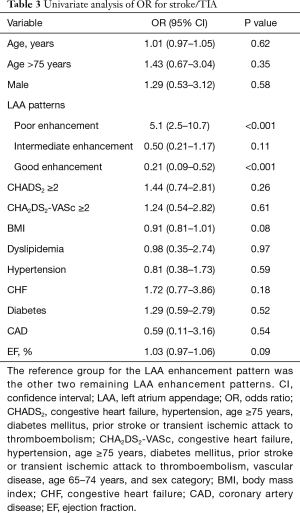
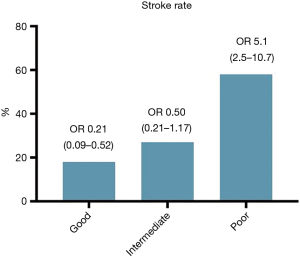
The variables previously known as risk factors were evaluated by a univariate analysis on the basis of having a history of stroke. The odds ratios (ORs) and 95% CIs for the baseline variables are shown in Table 3. Figure 4 shows that patients with a stroke history were more likely to have a poor enhancement pattern in the LAA than other patterns (OR: 5.1, 95% CI: 2.5–10.7, P<0.001). The CHA2DS2-VASc score was not a significant factor in this analysis.
Full table
Multivariate analysis
By adding factors previously regarded as stroke risk factors (CHA2DS2-VASc score, AF duration, congestive heart failure and ejection fraction) to the LAA patterns, a regression model was constructed with backward stepwise approach. The components of the CHA2DS2-VASc score were excluded because the correlation was significant. After controlling for the CHA2DS2-VASc score and ejection fraction using a multivariable logistic model, the poor enhancement pattern was found to be significantly more likely to be associated with a stroke history than the other patterns (OR: 5.3; 95% CI: 2.5–11.1; P<0.0001). This result did not change when the CHA2DS2-VASc score was replaced with the CHADS2 score (congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA).
LAA patterns and stroke risk in patients with low CHADS2/CHA2DS2-VASc score
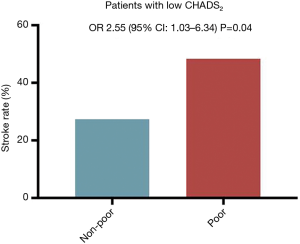
In 84 patients with a low CHADS2 score (0 or 1 point), those with a poor enhancement pattern had the highest risk of previous stroke (Figure 5). On a univariate analysis, only the poor enhancement pattern was identified as a risk factor of stroke (OR: 2.55, 95% CI: 1.03–6.34, P=0.04). The trend of results remained unchanged when replacing CHADS2 to CHA2DS2-VASc score, but statistically significance was not found. In 31 patients with a low CHA2DS2-VASc score (0 or 1 point), stroke history was more prevalent in patients with a poor enhancement pattern than in those with other patterns, but not statistically significant [6 of 21 (29%) vs. 5 of 10 (50%), P=0.06].
Discussion
In this study, different patterns categorized by contrast enhancement of medium in the LAA were correlated with the risk of prior stroke. We demonstrated that patients with a poor enhancement pattern or “pseudo-defect” had a statistically significant higher risk of prior stroke than those with other patterns. This result can be clinically useful, especially for patients with low CHA2DS2-VASc scores of 0 or 1, as these patients are generally judged to be in the low-risk group for stroke/TIA, but the presence of LAA pseudo-defect increases the risk of thromboembolism. This fact suggests that such patients, even those with a low CHA2DS2-VASc score, should be treated with anticoagulants. Furthermore, in patients with CHA2DS2-VASc scores of 1, anticoagulation therapy may not necessarily be needed if the LAA enhancement pattern is the good enhancement pattern. These present findings suggest the appropriateness of indications for anticoagulation therapy and may influence changes in the guidelines for AF treatment.
CHA2DS2-VASc score and LAA evaluation
The CHA2DS2-VASc score is the most prevalent and practical indicator for calculating the risk of cardiogenic thromboembolism in patients with AF. In the clinical guideline suggested by AHA/ACC (5), the need for anticoagulants in patients with CHA2DS2-VASc scores higher than 1 is outlined, but whether or not anticoagulation should be introduced for patients with low CHA2DS2-VASc scores, especially scores of 1, is controversial. Some patients with low CHA2DS2-VASc scores suffer from thromboembolism caused by LAA thrombus. In the evaluation of the CHA2DS2-VASc score, LAA characteristics are not taken into account.
Previous studies have shown that LAA characteristics are related to the risk of cardiogenic thromboembolism. Funabashi et al. reported that LAA CT contrast defects may be a prognostic indicator of thromboembolism in AF patients (28). However, they focused on CT attenuation occurring at just one point of the LAA while we investigated the entire LAA, but the concept and the results were concordant with our hypothesis. We also evaluated the CT contrast defect more comprehensively and identified three patterns. Kimura et al. reported that poor enhancement was not a factor relevant to stroke (15); however, in their study, this point was not a keynote, and their definition of poor enhancement is unclear. Yaghi et al. reported that the prevalence of non-chicken wing LAA morphology was higher among patients with cardiogenic stroke and embolic stroke of undetermined source than among those with non-cardiogenic stroke (29). The difference was not significant, but the trend was concordant to previous reports.
LAA blood flow evaluation
Under the existing guidelines for AF treatment, the decision to prescribe anticoagulants is made based on the CHA2DS2-VASc score, which accounts for just one factor of Virchow’s triad, the endothelial dysfunction. Our study may demonstrate that another factor of Virchow’s triad, blood flow, is also related to thrombus formation in LAA. This new factor is related to the stroke history independent of the CHA2DS2-VASc score. Various indicators related to the LAA have been suggested (13,30,31), but they represent the blood flow to the LAA indirectly. For example, complicated morphology may lead to congestion of blood flow, but other factors, such as the ejection fraction and heart rate, also affect the blood flow to the LAA. However, our new factor may represent the blood flow to the LAA directly.
Potential clinical implications
The occurrence of an insufficient blood flow when the contrast medium entered the LAA was observed.
These results include all factors concerning the blood flow, including the ejection fraction, heart rate, size, and morphology of the LAA, which may be the reason why a strong correlation between this factor and cardiogenic stroke history exists.
The simplicity of this indicator is also important. A tool that is difficult to use will not enjoy widespread adoption, so we tried to make the definition as clear and easy-to-understand as possible. The definitions of “poor enhancement” and “good enhancement” patterns are clear; the former requires only the presence of a linear border, and the latter requires only the same pattern as in the late phase. When the pattern does not fit either or when deciding if the pattern is poor or good is difficult, the pattern may be categorized as an intermediate risk pattern. The intermediate enhancement pattern is useful for enhancing the inter-observer reliability. In addition, the good kappa values among the operators indicate that the inter-observer variability with this method is minimized.
Study limitation
Several limitations associated with the present study warrant mention. First, this study is retrospective and includes a small sample size. Second, our data are derived from a population of patients who were scheduled to undergo surgery or catheter treatment, meaning that the rate of a stroke history was markedly higher than in the general population with AF. Third, the protocol and the performance of the device affect the results, which makes replication research difficult. A similar study with another protocol or device should be performed to validate our findings.
Conclusions
This study suggests that patients with a poor contrast medium enhancement pattern in the LAA have a significantly higher rate of cardiogenic stroke history than those with other patterns, after controlling for the CHA2DS2-VASc score.
Acknowledgments
We thank Motoyuki Hisagi and Toshiya Ohtsuka (at Tokyo Metropolitan Tama Medical Center) for cooperating with the data collection for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study obtained ethics approval of institutional review board (Tsukuba Memorial Hospital Ethics Committee, approval number H-27901), and the participants gave informed consent before taking part.
References
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [Crossref] [PubMed]
- Falk RH. Atrial fibrillation. N Engl J Med 2001;344:1067-78. [Crossref] [PubMed]
- Manning WJ, Silverman DI, Katz SE, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol 1994;23:1535-40. [Crossref] [PubMed]
- van den Ham HA, Klungel OH, Singer DE, et al. Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation: Results From a National Primary Care Database. J Am Coll Cardiol 2015;66:1851-9. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199-267. [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609-78. [Crossref] [PubMed]
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. [Crossref] [PubMed]
- Berti S, Pastormerlo LE, Rezzaghi M, et al. Left atrial appendage occlusion in high-risk patients with non-valvular atrial fibrillation. Heart 2016;102:1969-73. [Crossref] [PubMed]
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. [Crossref] [PubMed]
- Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. [Crossref] [PubMed]
- Inoue T, Suematsu Y. Left atrial appendage resection can be performed minimally invasively with good clinical and echocardiographic outcomes without any severe risk. Eur J Cardiothorac Surg 2018;54:78-83. [Crossref] [PubMed]
- Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531-8. [Crossref] [PubMed]
- Lee JM, Shim J, Uhm JS, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol 2014;113:963-9. [Crossref] [PubMed]
- Khurram IM, Dewire J, Mager M, et al. Relationship between left atrial appendage morphology and stroke in patients with atrial fibrillation. Heart Rhythm 2013;10:1843-9. [Crossref] [PubMed]
- Kimura T, Takatsuki S, Inagawa K, et al. Anatomical characteristics of the left atrial appendage in cardiogenic stroke with low CHADS2 scores. Heart Rhythm 2013;10:921-5. [Crossref] [PubMed]
- Nedios S, Koutalas E, Kornej J, et al. Cardiogenic Stroke Despite Low CHA2 DS2 -VASc Score: Assessing Stroke risk by Left Atrial Appendage Anatomy (ASK LAA). J Cardiovasc Electrophysiol 2015;26:915-21. [Crossref] [PubMed]
- Leung DY, Black IW, Cranney GB, et al. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol 1994;24:755-62. [Crossref] [PubMed]
- Jaber WA, Prior DL, Thamilarasan M, et al. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: A transesophageal echocardiographic study. Am Heart J 2000;140:150-6. [Crossref] [PubMed]
- Matyal R, Mahmood F, Chaudhry H, et al. Left atrial appendage thrombus and real-time 3-dimensional transesophageal echocardiography. J Cardiothorac Vasc Anesth 2010;24:977-9. [Crossref] [PubMed]
- Patel A, Au E, Donegan K, et al. Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm 2008;5:253-60. [Crossref] [PubMed]
- Jaber WA, White RD, Kuzmiak SA, et al. Comparison of ability to identify left atrial thrombus by three-dimensional tomography versus transesophageal echocardiography in patients with atrial fibrillation. Am J Cardiol 2004;93:486-9. [Crossref] [PubMed]
- Romero J, Husain SA, Kelesidis I, et al. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6:185-94. [Crossref] [PubMed]
- Kim YY, Klein AL, Halliburton SS, et al. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J 2007;154:1199-205. [Crossref] [PubMed]
- Martinez MW, Kirsch J, Williamson EE, et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging 2009;2:69-76. [Crossref] [PubMed]
- Bilchick KC, Mealor A, Gonzalez J, et al. Effectiveness of integrating delayed computed tomography angiography imaging for left atrial appendage thrombus exclusion into the care of patients undergoing ablation of atrial fibrillation. Heart Rhythm 2016;13:12-9. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Funabashi N, Takaoka H, Uehara M, et al. LAA CT contrast defects correlate with TEE LAA velocity and CHADS2-score and are a prognostic indicator for embolism in subjects with atrial fibrillation or flutter. Int J Cardiol 2015;185:297-300. [Crossref] [PubMed]
- Yaghi S, Chang AD, Hung P, et al. Left Atrial Appendage Morphology and Embolic Stroke of Undetermined Source: A Cross-Sectional Multicenter Pilot Study. J Stroke Cerebrovasc Dis 2018;27:1497-501. [Crossref] [PubMed]
- Anselmino M, Scaglione M, Di Biase L, et al. Left atrial appendage morphology and silent cerebral ischemia in patients with atrial fibrillation. Heart Rhythm 2014;11:2-7. [Crossref] [PubMed]
- Wang Y, Di Biase L, Horton RP, et al. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J Cardiovasc Electrophysiol 2010;21:973-82. [Crossref] [PubMed]


