
Validation of spectral energy for the quantitative analysis of ventricular fibrillation waveform to guide defibrillation in a porcine model of cardiac arrest and resuscitation
Introduction
Although incidence of ventricular fibrillation (VF) as a presenting rhythm are decreasing, it is still the initial rhythm of 19.5% of adult out-of-hospital cardiac arrest cases in the United States (1). Despite the fact that electrical defibrillation performed at an early stage is the best treatment for VF, research has shown that the number of defibrillation attempts should be minimized and that attempts with a low probability of return of spontaneous circulation (ROSC) should be delayed to allow for additional cardiopulmonary resuscitation (CPR) (2,3). Since defibrillation interrupts CPR that improve myocardial blood flow during VF, it may increase the severity of ischemic injury to organs and ineffective, unsuccessful, and repeated high-energy electrical shocks may damage myocardial function (4,5).
Recent studies have focused on optimizing the defibrillation timing during VF to improve the success of defibrillation (6,7). VF electrocardiograms (ECGs) are correlated with downtime and the myocardial metabolic state, and studies have shown that the quantitative waveform measures (QWM), non-invasive measurements derived from ECG characteristics (such as frequency and amplitude), can potentially predict defibrillation success and optimize defibrillation timing (7,8). The amplitude spectrum area (AMSA) is one of the waveform parameters that is calculated by the weighted area under the frequency spectrum, and is generally considered one of the most accurate predictors for successful defibrillation (9-12).
Although the mechanisms of the relationship between QWM and defibrillation success are not currently understood, clarifying the correlation between myocardial energy and the VF waveform metric may shed some light on the process (13). Prior research has suggested that a decrease in the VF waveform frequency is due to the loss of intramyocardial adenosine triphosphate concentration. A higher AMSA value has also been demonstrated to reflect higher myocardial energy stores that are more likely to aid in the successful progression of defibrillation to a perfusing rhythm (14,15). Various VF analysis strategies based on VF ECG traits such as frequency (AMSA), slope (median slope), or amplitude (Spectral Energy) have been investigated to optimize the timing of defibrillation and improve the outcome of VF (16-18). The power spectrum of a VF ECG might provide more details about the myocardial-consumed energy and could indirectly reflect the degree of myocardial metabolic activity during the VF process. In this study, we assessed the accuracy of the Spectral Energy method for predicting the success of defibrillation and compared it with the AMSA method to test the hypothesis that the Spectral Energy and AMSA methods have a similar ability to predict the success of defibrillation. In addition, we sought to determine the effects of epinephrine and CPR on AMSA and Spectral Energy.
Methods
Sixty male domestic pigs weighing 35 to 45 kg were involved in this study. All animal experiments were performed in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines (19). The protocol was approved by the Institutional Animal Care and Use Committee of the Tang Wanchun Laboratories of Emergency & Critical Care Medicine at Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
Animal preparation
Animals were fasted overnight but given free access to water. Anesthesia was initiated by an intramuscular injection of ketamine (20 mg/kg), followed by an intravascular injection of sodium pentobarbital (loading dose, 30 mg/kg). An additional dose of sodium pentobarbital (maintain dose, 8 mg/kg) was injected if animals awakened or showed signs of restlessness, and was repeated at intervals of approximately 1 h if necessary. The minimum time between the start of VF and last loading pentobarbital administration is at least 2 and 1 h for the last maintain administration. A cuffed endotracheal tube was introduced into the trachea and a VELA ventilator (CareFusion, California, US) was used with a tidal volume of 10 mL/kg body weight, a peak flow below 40 L/min, and 0.21 FiO2. A capnometer module of a BeneView T5 patient monitor (Mindray, Shenzhen, China) was used to measure end-tidal carbon dioxide pressure (ETCO2). Respiratory frequency was adjusted as necessary to maintain an ETCO2 between 35–45 mmHg. Body temperature was maintained at 37.5±0.5 °C throughout the entire experiment with the aid of a cooling/warming blanket (HGT-200II, Hokai Medical Instruments Corporation, Zhuhai, China). For the measurement of aortic pressure, a 6-F catheter was inserted into the thoracic aorta through the right femoral artery. A 7-F four-chambered Swan-Ganz catheter (774HF75 Swan-Ganz TD Catheter, Edwards Lifesciences Corporation, Irvine, CA, USA) was advanced from the right femoral vein into the right atrium for measurement of right atrium pressure.
Experimental procedures
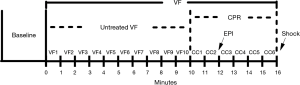
Fifteen min prior to the induction of VF, baseline measurements were obtained. VF was induced by a 2-mA alternating current through the pacing catheter in the right ventricular endocardium. Mechanical ventilation was discontinued after the onset of VF. After untreated VF was sustained for 10 min, two researchers initiated a two-person CPR algorithm of basic adult life support, as recommended by the 2015 American Heart Association (AHA) guidelines (20). The researchers provided high-quality CPR with 100 to 120 compressions per minute, allowing complete chest recoil and minimum interruption. The compression depth was ~25% of the anteroposterior diameter of the thorax. A feedback device (M-Series, Zoll medical corporation, Chelmsford, MA, USA) was used to monitor the rate and depth of chest compressions. Pure oxygen was delivered with a breathing bag linked to the endotracheal tube at a compression-ventilation ratio of 30:2. CPR was maintained for a total of 6 min. Two min after initiation of CPR, epinephrine at a dose of 20 µg/kg was administered to the animals. Six min after initiation of CPR, a single 120-J biphasic shock (M-Series, Zoll Medical Corporation, Chelmsford, MA, USA) was applied to terminate VF. If a rhythm with a mean aortic pressure of >50 mmHg persisted for an interval of 5 mins or more, it was regarded as ROSC. This timeline is shown in Figure 1.
General measurements
Hemodynamic data and ECG were continuously measured and recorded through a data acquisition system supported by Windaq hardware/software (Dataq Instruments Inc., Akron, OH, USA). The coronary perfusion pressure (CPP) was digitally computed from the differences in time-coincident diastolic arterial pressure and right atrium pressure.
Spectral energy and AMSA methods
The experimental data was reviewed to assess the accuracy of the Spectral Energy method for predicting the success of defibrillation, and then compared with the AMSA method. ROSC was regarded as the sign of defibrillation success. MATLAB 2014a (The Mathworks, Natick, MA, USA) was used for all analysis. The one-second electrocardiographic lead II recordings that immediately preceded the first electrical shock were analyzed for each measurement. The success of defibrillation was recorded. ECG recordings during pauses in chest compressions were processed using a bandpass filter between 4 and 48 Hz to eliminate the high frequency noise produced by the power line and myoelectrical activity. Then, the one-second ECG segment was transformed from the time to the frequency domain using the Fast Fourier Transform. The Spectral Energy was calculated according to the following equation: 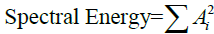 , where Ai is the amplitude corresponding to the ith frequency fi in the ECG. The AMSA value of each one-second ECG segment was also calculated according to the equation:
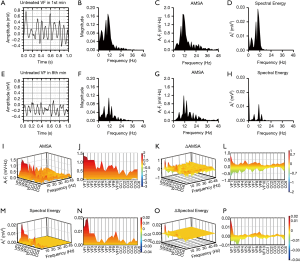
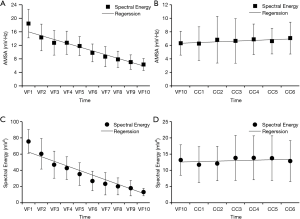
, where Ai is the amplitude corresponding to the ith frequency fi in the ECG. The AMSA value of each one-second ECG segment was also calculated according to the equation: 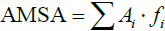 . In this study, the same frequency band between 4 and 48 Hz was analyzed for both methods. Figure 2A,B,C,D,E,F,G,H shows the process by which the Spectral Energy and AMSA values were calculated, where the subplots from Figure 2 show a one-second ECG segment in the 1st min (8th min) of the untreated VF phase, the amplitude spectrum, the calculated AMSA spectrum, and the Spectral Energy spectrum.
. In this study, the same frequency band between 4 and 48 Hz was analyzed for both methods. Figure 2A,B,C,D,E,F,G,H shows the process by which the Spectral Energy and AMSA values were calculated, where the subplots from Figure 2 show a one-second ECG segment in the 1st min (8th min) of the untreated VF phase, the amplitude spectrum, the calculated AMSA spectrum, and the Spectral Energy spectrum.
Statistical analyses
Sample size was at least 60 animals with α=0.05, two-tailed and a power of 80%. Power analysis was done to calculate the sample size using PASS 11 software (NCSS, Kaysville, UT, USA) before the study. Animals were categorized into two groups: Group R, where the animal’s single defibrillation was successful; and Group N, where the animal’s single defibrillation failed. The AMSA and Spectral Energy values were calculated for samples in each group. Continuous variables were shown as mean ± standard deviation (SD) if data was normally distributed. If data was not normally distributed, a median (25th, 75th percentiles) were shown. Normal distribution was confirmed by the Kolmogorov-Smirnov test. Comparisons between time-based measurements within each group were performed with one-way analyses of variance. Differences of AMSA or Spectral Energy between CC1 to CC6 and VF10 were calculated, which were defined as ΔAMSA and ΔSpectral Energy, respectively. For example, at the CC1 timepoint, the value of ΔAMSA is the AMSA value of CC1 minus the AMSA value of VF10. Next, the ΔAMSA and ΔSpectral Energy values of Group R were compared with Group N. Finally, receiver operating characteristic (ROC) curves were generated for the Spectral Energy and AMSA methods. The areas under the ROC curves (AUCs) were calculated for both methods and compared using a two-tailed Z-test implemented in MedCalc 15 software (MedCalc Software, Mariakerke, Belgium) (21). The MedCalc 15 software was also used to calculate 95% CIs (two-tailed) for AMSA and Spectral Energy values at CC6. For all analyses, P<0.05 was considered to be statistically significant.
Results
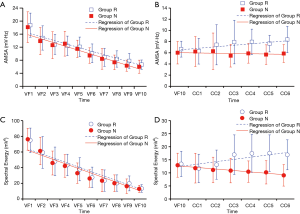
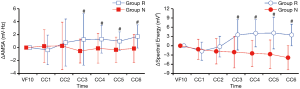
Among the 60 animals included in this observational study, successful defibrillation was achieved for 29 (48%), and failed in 31 (52%). Accordingly, Group R and Group N were composed of 29 and 31 animals, respectively. There were no significant differences in baseline physiologies between both Groups R and N (Table 1). Although no obvious difference in the CPP at 1st min was observed between both groups, CPPs at 3rd and 5th min were significantly greater in Group R (Table 1). As shown in Figure 3, the AMSA and Spectral Energy values decayed over time during untreated VF (18.44±4.23 vs. 6.32±1.77 mV/Hz, P<0.001; 75.61±14.65 vs. 13.17±4.69 mV2, P<0.001). Although the AMSA and Spectral Energy values of the animals in Group R increased after the implementation of CPR and epinephrine (6.29±2.30 vs. 8.37±2.36 mV/Hz, P=0.001; 11.68±5.45 vs. 16.91±5.72 mV2, P=0.001), no changes in ECG were observed in animals from Group N (Figure 4). The ΔAMSA and ΔSpectral Energy of Group R were significantly higher than Group N after two min of CPR (1.26±3.97 vs. ‒0.53±1.85, P<0.05, 3.54±4.92 vs. ‒2.07±5.13, P<0.05), as shown in Figure 5.
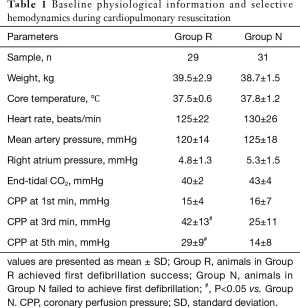
Full table
The AUCs of the AMSA and Spectral Energy methods at CC3, CC4, CC5, and CC6 were 0.76 vs. 0.74, 0.78 vs. 0.81, 0.81 vs. 0.82, and 0.81 vs. 0.88, respectively (Figure 6). Both methods were significantly predictive of outcome (P<0.01 in all cases). The prognostic characteristics of both methods in terms of sensitivity and specificity were similar, and the ROCs of both methods at each timepoint showed no significant differences (Table 2). There were no significant differences in the ROCs between different timepoints of both methods. The cut-off values of the AMSA and Spectral Energy methods at CC6 were 6.95 and 10.21, respectively. The CI of AMSA values at CC6 is (2.46, 11.68) while the CI of Spectral Energy values is obtained as (1.97, 30.44).
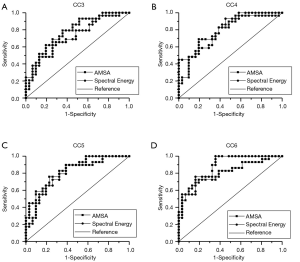
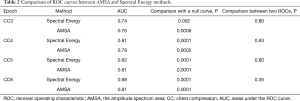
Full table
Discussion
In the present study, it was found that Spectral Energy and AMSA values decreased over time during untreated VF. As shown in Figure 3, after ten min of untreated VF, AMSA values decreased from 18.44±4.23 to 6.32±1.77 mV/Hz, while Spectral Energy values decreased from 75.61±14.65 to 13.17±4.69 mV2. However, increases in the mean Spectral Energy and the mean AMSA values after the application of CPR and epinephrine were observed in successfully resuscitated animals, in contrast to non-responsive animals, indicating that this change in AMSA and Spectral Energy over time is an indicator of a good outcome. Moreover, the Spectral Energy and AMSA methods had a similar ability to predict the success of defibrillation.
The results of recent studies have shown that Spectral Energy is one of the characteristic patterns of the VF ECG signal that can be used to estimate the duration of VF (16). It can be seen in heat maps (Figure 2) that the main components of Spectral Energy and AMSA are in the low frequency band, in which the dominant frequencies of Spectral Energy are concentrated in the range of 5 to 20 Hz, while the dominant frequencies of AMSA are concentrated from 5 to 25 Hz. These results were consistent with the characteristic of AMSA. Since AMSA is frequencies sensitive, high frequency components with low amplitude may have an impact on AMSA values (22). However, it has been suggested that filtering out frequency components with low amplitude improves performance of AMSA method (23). Therefore, Spectral Energy method which is high amplitude dominant may have an inherent advantage. Both AMSA and Spectral Energy values showed a significant decline during VF. From a physiological point of view, the metabolic level of the heart during VF is typically in a downward trend (24). The observed declines in AMSA and Spectral Energy during untreated VF are consistent with a downward metabolic trend in the myocardium.
During VF, if no rescue measures such as CPR or defibrillation are applied, the energy of cardiomyocytes gradually decays until exhaustion, and the depletion of myocardial energy during VF has been shown to correlate with QWM (13,14). In this study, the Spectral Energy and AMSA values decreased during the untreated VF phase in a time-dependent manner. Both the Spectral Energy and AMSA values in the successfully defibrillated animals increased again when CPR and epinephrine were applied, but no responses to CPR and epinephrine were observed in animals in which defibrillation failed. Previous research has also shown that AMSA increases after effective CPR (25) and its value is associated with the concentration of adenosine triphosphate (3,14). The Spectral Energy method, which could indirectly reflect myocardial metabolic activity, might also serve as a predictive indicator for effectiveness of CPR, revealing whether the myocardial blood flow has been effectively improved. However, all animals in the present experiment were also administered epinephrine during resuscitation. For this reason, it is difficult to distinguish the effects of CPR from epinephrine. The effect of repeated epinephrine doses on AMSA values was described in a study by Wagner et al., but this prior study did not compare the differences between a relative Group R and Group N (26). In their trials, there was no statistical difference in AMSA values calculated from animals that received epinephrine versus animals that did not receive epinephrine. In our study, the ΔAMSA and ΔSpectral Energy of Group R were always greater than zero after two min of CPR where epinephrine was administered, which means that the Spectral Energy and AMSA values in animals which were successfully defibrillated significantly increased. In contrast, the ΔAMSA and ΔSpectral Energy values of Group N were smaller than zero, and were significantly less than Group R. These results suggest that epinephrine is effective and important for long-term resuscitation, in line with the 2015 AHA guidelines (20). Furthermore, we speculate that the values of AMSA or Spectral Energy from ECG may be suitable guides to predict the effect of CPR on outcomes. For example, during resuscitation, if AMSA or Spectral Energy values increase after the implementation of CPR and epinephrine, a subsequent defibrillation may produce a better result versus a case in which AMSA or Spectral Energy values decrease after implementation. Moreover, the possibility of successful defibrillation can be predicted by analyzing the change in Spectral Energy or AMSA after the initiation of CPR.
We also found that higher Spectral Energy and AMSA values were correlated with defibrillation success. As previously demonstrated, AMSA effectively predicted the success of defibrillation and the possibility of ROSC (5,27-29). Thus, the Spectral Energy method could also be a valuable parameter for predicting the outcome of defibrillation (16). The ROC curve results further confirmed the ability of the Spectral Energy method to predict successful defibrillation. Among all the QWM used to determine the timing of defibrillation, AMSA is generally considered one of the most accurate predictors for successful defibrillation (9,15), and the predictive sensitivity and specificity values between Spectral Energy and AMSA were similar. Therefore, the Spectral Energy method could be an alternative predictor for successful defibrillation. Since the formula of Spectral Energy is consistent with the signal energy theory of signal processing, it may be beneficial to predict successful defibrillation using Spectral Energy method.
There were several limitations to our study. First, this is a relatively small animal study, and a study with a larger sample number should also be performed. In addition, the species difference between pigs and humans should be noted, and thus further work is needed to validate our findings for human cardiac arrest. Second, the VF waveform segment collected during ventilations was analyzed in this study, which did not include chest compressions. Nevertheless, only one-second ECG segments were used, a pause which was acceptable and had little effect on the quality of CPR. Third, all animals in this experiment were administered epinephrine during resuscitation. Experiments with a control group that does not receive epinephrine should be conducted in the future. Fourth, the investigation was an observational animal study. Ideally, we would further explore the ability of the threshold value in the Spectral Energy method to optimize the defibrillation time and predicting the possibility of ROSC in a prospective randomized controlled trial.
Conclusions
Both the Spectral Energy and AMSA methods accurately predict the success of defibrillation. Moreover, increases in the value of either Spectral Energy or AMSA after application of CPR and epinephrine may also predict successful defibrillation.
Acknowledgments
Funding: This work was supported by research grants from the projects of Leading Talents in Pearl River Talent Plan of Guangdong Province (No. 81000-42020004), and Natural Science Foundation of Guangdong Province (No. 2014A030313510 and No. 2018A0303113540).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol was approved by the Institutional Animal Care and Use Committee of the Tang Wanchun Laboratories of Emergency & Critical Care Medicine at Sun Yat-sen Memorial Hospital, Sun Yat-sen University (P1610).
References
- Soar J, Donnino MW, Maconochie I, et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Resuscitation 2018;133:194-206. [Crossref] [PubMed]
- Lee JC, Suh GJ, Kim HC. Electrocardiogram frequency change by extracorporeal blood perfusion in a swine ventricular fibrillation model. Biomed Eng Online 2013;12:123. [Crossref] [PubMed]
- Nakagawa Y, Amino M, Inokuchi S, et al. Novel CPR system that predicts return of spontaneous circulation from amplitude spectral area before electric shock in ventricular fibrillation. Resuscitation 2017;113:8. [Crossref] [PubMed]
- Affatato R, Li Y, Ristagno G. See through ECG technology during cardiopulmonary resuscitation to analyze rhythm and predict defibrillation outcome. Curr Opin Crit Care 2016;22:199-205. [Crossref] [PubMed]
- Nakagawa Y, Sato Y, Kojima T, et al. Electrical defibrillation outcome prediction by waveform analysis of ventricular fibrillation in cardiac arrest out of hospital patients. Tokai J Exp Clin Med 2012;37:1-5. [PubMed]
- Gong Y, Lu Y, Zhang L, et al. Predict Defibrillation Outcome Using Stepping Increment of Poincare Plot for Out-of-Hospital Ventricular Fibrillation Cardiac Arrest. Biomed Res Int 2015;2015:493472. [Crossref] [PubMed]
- He M, Chen B, Gong Y, et al. Prediction of Defibrillation Outcome by Ventricular Fibrillation Waveform Analysis: A Clinical Review. J Clinic Experiment Cardiol 2013;S10:009.
- Coult J, Sherman L, Kwok H, et al. Short ECG segments predict defibrillation outcome using quantitative waveform measures. Resuscitation 2016;109:16-20. [Crossref] [PubMed]
- Coult J, Kwok H, Sherman L, Blackwood J, et al. Ventricular fibrillation waveform measures combined with prior shock outcome predict defibrillation success during cardiopulmonary resuscitation. J Electrocardiol 2018;51:99-106. [Crossref] [PubMed]
- Jin D, Dai C, Gong Y, et al. Does the choice of definition for defibrillation and CPR success impact the predictability of ventricular fibrillation waveform analysis? Resuscitation 2017;111:48-54. [Crossref] [PubMed]
- Marn-Pernat A, Weil MH, Tang W, et al. Optimizing timing of ventricular defibrillation. Crit Care Med 2001;29:2360-5. [Crossref] [PubMed]
- Povoas HP, Bisera J. Electrocardiographic waveform analysis for predicting the success of defibrillation. Crit Care Med 2000;28:N210-1.
- Salcido DD, Menegazzi JJ, Suffoletto BP, et al. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation 2009;80:946-50. [Crossref] [PubMed]
- Neumar RW, Brown CG, Van Ligten P, et al. Estimation of myocardial ischemic injury during ventricular fibrillation with total circulatory arrest using high-energy phosphates and lactate as metabolic markers. Ann Emerg Med 1991;20:222-9. [Crossref] [PubMed]
- Ristagno G, Mauri T, Cesana G, et al. Amplitude spectrum area to guide defibrillation: a validation on 1617 patients with ventricular fibrillation. Circulation 2015;131:478-87. [Crossref] [PubMed]
- Eftestol T, Sunde K, Ole AS, et al. Predicting outcome of defibrillation by spectral characterization and nonparametric classification of ventricular fibrillation in patients with out-of-hospital cardiac arrest. Circulation 2000;102:1523. [Crossref] [PubMed]
- Firoozabadi R, Nakagawa M, Helfenbein ED, et al. Predicting defibrillation success in sudden cardiac arrest patients. J Electrocardiol 2013;46:473-9. [Crossref] [PubMed]
- He M, Gong Y, Li Y, et al. Combining multiple ECG features does not improve prediction of defibrillation outcome compared to single features in a large population of out-of-hospital cardiac arrests. Crit Care 2015;19:425. [Crossref] [PubMed]
- Kilkenny C, Browne W, Cuthill IC, et al. Animal Research: Reporting In Vivo Experiments: The ARRIVE Guidelines. The Journal of Gene Medicine 2010;12:561-3. [Crossref] [PubMed]
- Travers AH, Perkins GD, Berg RA, et al. Part 3: Adult Basic Life Support and Automated External Defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015;132:S51-83. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Ng J, Goldberger JJ. The ups and downs of ventricular fibrillation waveforms. J Am Coll Cardiol 2014;64:1370-2. [Crossref] [PubMed]
- Xie Z, Yang Q, Li M, et al. Amplitude screening improves performance of AMSA method for predicting success of defibrillation in swine model. Am J Emerg Med 2019;37:1224-9. [Crossref] [PubMed]
- Sherman LD, Flagg A, Callaway CW, et al. Angular velocity: a new method to improve prediction of ventricular fibrillation duration. Resuscitation 2004;60:79-90. [Crossref] [PubMed]
- Schoene P, Coult J, Murphy L, et al. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Heart Rhythm 2014;11:230-6. [Crossref] [PubMed]
- Wagner H, Gotberg M, Madsen Hardig B, et al. Repeated epinephrine doses during prolonged cardiopulmonary resuscitation have limited effects on myocardial blood flow: a randomized porcine study. BMC Cardiovasc Disord 2014;14:199. [Crossref] [PubMed]
- Li Y, Ristagno G, Bisera J, et al. Electrocardiogram waveforms for monitoring effectiveness of chest compression during cardiopulmonary resuscitation. Crit Care Med 2008;36:211. [Crossref] [PubMed]
- McGovern M, Allen D, Chaudhry F, et al. The ventricular fibrillation waveform approach to direct postshock chest compressions in a swine model of VF arrest. J Emerg Med 2015;48:373-81. [Crossref] [PubMed]
- Salcido DD, Kim YM, Sherman LD, et al. Quantitative waveform measures of the electrocardiogram as continuous physiologic feedback during resuscitation with cardiopulmonary bypass. Resuscitation 2012;83:505-10. [Crossref] [PubMed]


