NF-κB1 promoter-94ins/delATTG polymorphisms correlate with the acute exacerbation of chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a major threat to human health. Epidemiological data (1) shows that the prevalence of COPD patients aged above 40 years old in China is 8.2% and each year COPD patients encounter acute exacerbation (AE) on 0.5 to 3.5 occasions. Studies (2) have confirmed that acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is an important cause of hospitalization and death in patients with COPD. This disease represents a serious economic and social burden.
The pathogenesis of COPD remains unclear but is known to involve environmental and genetic factors (3) and inflammatory cells, inflammatory mediators and cytokines. As a pivotal signaling pathway, NF-κB plays an important role in COPD occurrence. Pierrou et al. (4) revealed that the bronchial epithelial cells of COPD patients overexpress a large number of transcription factors including NF-κB. Brown et al. (5) found that the excessive expression or activation of NF-κB1 in patients with COPD and lung cancer led to exacerbated inflammatory responses, making COPD patients more prone to lung cancer.
It is known now that an ATTG insertion or deletion (ins/del) at the 94 bp region upstream of the NF-κB p50 (NF-κB1 gene) transcription initiation site influences COPD development. Studies (6) have shown that the NF-κB1 gene initiation sequence -94ins/delATTG polymorphism influences the expression of NF-κB which in turn affects disease progression. Others (7) have confirmed that the NF-κB1 gene initiation sequence -94ins/delATTG represents a susceptibility gene for chronic lung disease, but the correlation between NF-κB1 gene polymorphisms and the AE of chronic lung disease has not been defined.
In this study, we used polymerase chain reaction high resolution melt (PCR-HRM) to assess the distribution of NF-κB1 gene initiation sequence -94ins/delATTG polymorphisms amongst the Chinese Han population with COPD. We further assessed the relationship between polymorphisms and the AECOPD.
Methods
Subjects
A total of 260 patients with COPD were recruited from the outpatient and inpatient clinics of the Department of Respiratory Medicine of the Third Affiliated Hospital of the Southern Medical University during September 2013 to September 2015. Patients were diagnosed as COPD by following the global initiative for chronic obstructive lung disease (GOLD) diagnostic criteria (3).
The diagnostic criteria were as follows: AE diagnosis criteria: COPD patients diagnosed when their respiratory symptoms continued to deteriorate abnormally with a requirement for other medication regimens (e.g., fluticasone/salmeterol in stable COPD; intravenous hexadecadrol for AECOPD). During disease progression, patients often present with short-term coughs, expectoration, a shortness of breath, wheezing phlegm, increased pus and phlegm or mucus purulent sputum accompanied by fever and other aggravations. A total of 260 healthy subjects were selected as the control group. Body mass index (BMI) and COPD assessment test (CAT) scores were assessed in all patients.
Inclusion criteria
Met the above diagnostic criteria; unrelated; provided informed consent and cooperated with all experiments.
Exclusion criteria
Patients with respiratory system diseases such as bronchiectasis, pulmonary fibrosis, sarcoidosis, bronchial asthma and inherited diseases such as diabetes, heart disease and rheumatic arthritis.
All selected patients and healthy controls were authorized by the Ethics Committee of clinical trials of the Third Affiliated Hospital of Southern Medical University (2016-clinic-042).
Reagents and instruments
DNA extraction kits were purchased from Fermentas Co., (K0512, Lithuania); Type-it HRM PCR kits were purchased from Qiagen Co., (206542, Germany). Rotor-Gene Q Analyzer kits were purchased from Qiagen Co., (Rotor-Gene Q, Germany). DNA quantitative analyzer kits were purchased from Fermentas Co., Lithuania.
Materials and methods
Genomic DNA extraction and quantification
A total of 1 mL of EDTA anticoagulant venous blood was collected from both patient and control groups. DNA was extracted using genomic DNA extraction kits according to the manufacturer’s instruction. The concentration and purity of the extracted genomic DNA were analyzed on a DNA quantitative analyzer. DNA samples were diluted to 30 ng/µL and stored at –20 °C.
PCR-HRM was used to detect NF-κB1 gene initiation sequences and regional polymorphisms. PCR sequences were as previously described (8). Upstream primer: 5'-CATGACTCTATCAGCGGCACT-3', downstream primer: 5'-GGCTCTGGCTTCCTAGCAG-3'; product length: 152 bps. Primers were synthesized by Weijiki (Shanghai) Trading Co., Ltd. PCRs were performed using Type-it HRM PCR kits. PCR reaction conditions were as follows: initial denaturation for 5 min at 96 °C; denaturation for 20 s at 96 °C; 20 s annealing at 57 °C; extension for 20 s at 72 °C; 40 cycles. HRM analysis and PCR reactions were performed simultaneously. HRM analysis conditions: 95 °C for 5 min; 40 °C for 2 min; 60 °C for l min, and collect data of melting curves at 0.1 °C/s from 72 °C to 95 °C. Each HRM analysis assessed the ins/ins and del/del genotypes simultaneously. Rotor-Gene 6000 1.7 was used to analyze the real-time data and determine the genotype. A total of 5% of the samples were randomly selected for direct sequencing to verify the HRM technology.
Statistical analysis
Data were processed using SPSS 20.0. The χ2 test was for group comparisons of the counting data. When the theoretical frequency was <5, a Fisher’s exact test was used for comparisons. Numerical data are presented as mean ± standard deviation (SD) (
Results
General information
No significant differences in age, sex and location were observed between the AECOPD and control groups (P>0.05, Table 1). The median smoking index (per cigarette/1 year) amongst the control group was 301.53±287.59 (pack/years).

Full table
NF-κB1 gene initiation sequence regional polymorphism analysis
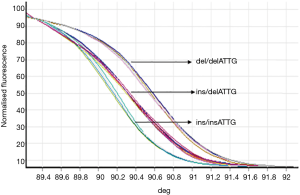
Samples from experimental and control groups were compared for NF-κB1 activation by PCR-HRM. The results showed that both the AECOPD and healthy control group showed detectable ins/ins, ins/del and del/del genotypes (Figures 1,2). Genetic balance analysis showed that the distribution of the three genotypes were consistent with the Hardy-Weinberg equilibrium law in both groups. The number of patients who carried ins/ins, ins/del and del/del genotypes were 93, 138 and 29 in the AECOPD group and 102, 106 and 52 in the control group respectively. The different distributions of the two groups were statistically significant (P<0.05, Table 2). Further analysis showed that the distribution of the composition ratio of ins/ins + ins/del and del/del between groups were statistically significant (P<0.05, Table 2). However, the distribution of the composition ratio of ins and del alleles did not significantly differ between groups (P>0.05, Table 2).

Full table
Correlation analysis of NF-κB1 gene initiation -94ins/delATTG and AECOPD
General information of the AECOPD patients
General information analysis showed no significant differences between ins/ins + ins/del and del/del in terms of patient gender, age and native location (P>0.05, Table 3).

Full table
Analysis of the clinical features of the ins/ins + ins/del and del/del genes in AECOPD patients
Whether the AECOPD patients carried ins/ins + ins/del or del/del genes were next assessed. The distribution composition was independent of the smoking index and clinical phenotype (P>0.05) as the control group had the same smoking history. AECOPD patients who carried the ins/ins + ins/del gene had a lower BMI, higher CAT scores, a higher number of acute episodes and longer hospital stays than the AECOPD patients with the del/del gene (P<0.05, Table 4).
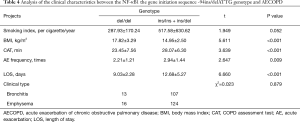
Full table
Discussion
A large number of studies (5,9-10) have shown that NF-κB is associated with COPD associated airway inflammation, which regulates the expression of pro-inflammatory genes that regulate cell proliferation, apoptosis and immune responses. Activated NF-κB delays neutrophil apoptosis and enhances the inflammatory response. In this study, ins/ins, ins/del and del/del were detected from AECOPD patients the majority of which carried ins/ins and ins/del. These results were consistent with studies by Huang and colleagues (11) who found that the lung function of the Chinese Han population with -94ins/insATTG genes or ins/delATTG genes showed reduced lung function (FEV1) that those carrying del/delATTG genes. The results of this study indicated that the COPD patients with -94ins/insATTG or -94ins/delATTG gene sequences had more severe disease with longer hospital stays. It is speculated that carrying the -94insattg gene increases the expression of NF-κB and influences the intensity of the inflammatory response. Karban et al. (6) confirmed that the NF-κB1 gene initiation sequence -94insATTG could increase the transcription capacity of NF-κB1 by ~2-fold, whilst the delATTG sequence reduced the transcription intensity of NF-κB1 in vitro. Adamzik et al. (12) found that the gene initiation sequence of NF-κB1 (-94ins/delATTG) was closely related to the severity of acute respiratory distress syndrome (ARDS), and patients with ARDS with -94del/delATTG genes had a lower incidence of lung injury, indicating poor prognosis.
We observed no differences in terms of smoking history (pack years) between those carrying del/del genotypes and those carrying ins/ins +ins/del genotypes. However, Szulakowski et al. (13) showed that the transcription of NF-κB in lung tissue and phlegm of COPD patients was higher than non-smokers. The various oxides in cigarettes activate NF-κB and AP-1 signaling to induce the expression of various cytokines and participate in the inflammatory processes of lung tissue. The results of the study were inconsistent with previous proteomic analysis, and thus require further verification (14).
We found that the BMI was an important indicator of the long-term prognosis of patients with COPD and thus an indicator of long-term survival. Patients with a low BMI tended to display more severe symptoms and higher mortality (15). We found that the BMI of patients with AECOPD was significantly lower than patients with the -94insATTG gene. We hypothesized that a correlation between body weight and the -94insATTG gene exists, which requires further confirmation.
Conclusions
We found that the NF-κB1 initiation sequence -94ins/delATTG is a susceptible gene for the development of AECOPD. As a result, monitoring the polymorphism permits the ability to prevent the AECOPD, providing a theoretical basis for individualized treatments. However, the sample size of this study was small and studies with multi-center large sample sizes in combination with proteomic analysis are required to confirm the role of the NF-κB1 gene initiation sequence -94ins/delATTG during the AECOPD.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All selected patients and healthy controls were authorized by the ethics committee of clinical trials of the third affiliated hospital of Southern Medical University (2016-clinic-042).
References
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [Crossref] [PubMed]
- Perera PN, Armstrong EP, Sherrill DL, et al. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD 2012;9:131-41. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013. [Crossref] [PubMed]
- Pierrou S, Broberg P, O’Donnell RA, et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:577-86. [Crossref] [PubMed]
- Brown V, Elborn JS, Bradley J, et al. Dysregulated apoptosis and NFkappaB expression in COPD subjects. Respir Res 2009;10:24. [Crossref] [PubMed]
- Karban AS, Okazaki T, Panhuysen CI, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet 2004;13:35-45. [Crossref] [PubMed]
- Korytina GF, Akhmadishina LZ, Kochetova OV, et al. Inflammatory and immune response genes polymorphisms are associated with susceptibility to chronic obstructive pulmonary disease in Tatars population from Russia. Biochem Genet 2016;54:388-412. [Crossref] [PubMed]
- Santos DG, Resende MF, Mill JG, et al. Nuclear factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med Genet 2010;11:89. [Crossref] [PubMed]
- Imanifooladi AA, Yazdani S, Nourani MR. The role of nuclear factor-kappaB in inflammatory lung disease. Inflamm Allergy Drug Targets 2010;9:197-205. [Crossref] [PubMed]
- Liu FT, Jia L, Wang P, et al. STAT3 and NF-κB cooperatively control in vitro spontaneous apoptosis and poor chemo-responsiveness in patients with chronic lymphocytic leukemia. Oncotarget 2016;7:32031-45. [PubMed]
- Huang D, Yang L, Liu Y, et al. Functional polymorphisms in NFκB1/IκBα predict risks of chronic obstructive pulmonary disease and lung cancer in Chinese. Hum Genet 2013;132:451-60. [Crossref] [PubMed]
- Adamzik M, Frey UH, Rieman K, et al. Insertion/deletion polymorphism in the promoter of NFKB1 influences severity but not mortality of acute respiratory distress syndrome. Intensive Care Med 2007;33:1199-203. [Crossref] [PubMed]
- Szulakowski P, Crowther AJ, Jiménez LA, et al. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:41-50. [Crossref] [PubMed]
- MacNee W. Oxidants/antioxidants and chronic obstructive pulmonary disease: pathogenesis to therapy. Novartis Found Symp 2001;234:169-85; discussion 185-8. [PubMed]
- Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J 2008;31:492-501. [Crossref] [PubMed]