Differences in the clinical and radiological characteristics of lung-involved toxocariasis between toxocariasis with eosinophilia and those without eosinophilia
Introduction
In the past, majority of the people in Korea were infected with parasites. But today, parasite infection rates have decreased. In 1971, 84.3% of the people were tested positive for parasite eggs, but by 1997, it had decreased to 2.4% (1). However, as the socio-economic status in Korea has improved and family structures have transformed into two-generation families, pets have become more popular (2) and parasitic disease levels have risen again. In an investigation of parasite infection in 1992, 297 (59%) among 503 dog stool samples contained more than one parasite, and Toxocara canis (T. canis) was present in about 11.1% (3). There are no reports of worldwide human T. canis infection rate. But T. canis infection of dogs has been reported worldwide, and canine infection rates widely vary. The environmental contamination with T. canis eggs in public grounds is also widespread (4-7).
The prevalence of toxocariasis is widely known to be high in children living in developing countries, particularly in schools and apartment playgrounds where contamination with parasite eggs can be up to 87% (8). Recently, adult infection with T. canis or Toxocara cati (T. cati) has been increasing. The reason seems to be the increase in overseas travels and the number of pet animals.
In Korea, 68% of the cases of eosinophilia are caused by toxocariasis, which indicates that T. canis infection is widespread among the Korean adults (9). There are a wide variety of involved organs, and 20% of the patients infected with T. canis or T. cati have invasive lung disease (10,11).
Toxocariasis is an illness of humans caused by larvae (immature worms) of either T. canis or T. cati. Toxocariasis is known to cause visceral larva migrans. T. canis and T. cati are parasites that live in the small intestines of dogs and cats. Mature animals become infected by eating eggs from the infected soil, and it is possible to acquire infection via vertical transmission from their mother’s milk or placenta. Parasitic eggs enter the soil from dog or cat feces. They enter the human intermediate host, in which the infected larvae penetrate the intestinal wall and migrate to organs including the liver, lungs, and eyes. This is known as visceral larva migrans; and if the skin is invaded, it is called cutaneous larva migrans. All of these are known as toxocariasis (12,13).
Kim et al. (11) characterized lung disease from toxocariasis in patients with eosinophilia, and reported an eosinophilic lung disease by toxocariasis rate of 23% (32/141 patients). Kwon et al. (9) reported a prevalence of 68% toxocariasis in patients with eosinophilia, and 24% had lung invasion. In a Korean study of 102 patients with toxocariasis lung invasion, only 70% were accompanied by eosinophilia (14). Therefore, we assume that 20-30% of patients with toxocariasis do not have eosinophilia. However, in the relation to the radiologic characteristics of patients without eosinophilia, no studies prior to the current study have analyzed large number of subjects and subtypes of ground glass opacity (GGO).
We studied patients who were tested positive for antibodies against Toxocara and who were diagnosed with parasitic lung diseases by chest computed tomography (CT), and the clinical and radiological features were compared between those with and without eosinophilia.
Patients and methods
Patients
From October 2009 to February 2014, we investigated patients from Chungnam National University Hospital, who were tested positive for antibodies against Toxocara by using enzyme-linked immunosorbent assay (ELISA) and who seemed to have infectious lung disease based on chest CT, during the admission or in the Outpatient Department. A total of 88 patients met eligible criteria to be included. We also measured IgG antibodies to Clonorchis sinensis, paragonimiasis, sparganosis, and cysticercosis antigens using ELISA in these patients, and the patients who had positive results were removed from the study. Therefore, we ruled out other parasitic infections. We performed retrospective analyses of the clinical outcomes, symptoms, chest CT, and initial laboratory data. We compared the radiological features in patients with toxocariasis and invasive lung disease with and without eosinophilia. Eosinophilia is defined by blood test showing greater than 500 cells/µL in the peripheral blood or 10% of total white blood cell count (15). Serum toxocara ELISA test was carried out based on the decision of the physician whether parasitic infection was suspected from clinical symptoms, history. Therefore, we tried to establish symptoms and hematological findings of toxocariasis with pulmonary invasion.
Diagnosis of toxocariasis larval infection
We measured antibodies against Toxocara using ELISA for serological testing of parasite antigens. Negative and positive serologic tests were defined as values below 0.250 and above 0.250, respectively. Serum was sent to the Institute of Infectious Disease/Department of Parasitology at College of Medicine in Seoul National University. The same samples were tested twice and the average values were derived. This method for measuring IgG antibodies against Toxocara has been reported to have 91% sensitivity and 86% specificity (16).
Data analysis methods
The blood tests were analyzed on the day of diagnosis. Many Koreans eat various forms of raw meat, and thus we also surveyed on the consumption of raw meat during the past 6 months. Chest CT findings of pulmonary involvement of toxocariasis were analyzed according to the methods of Cheon et al. (17) and Johkoh et al. (18). The findings were categorized into pulmonary infiltration pattern, anatomical distribution, and zonal distribution. The pulmonary infiltration category is shown by the plus notation if each lesions of different pattern can be seen in chest CT of a single patient. If there is a GGO lesion, the GGO was classified based on its main subtype, according to Suzuki et al. (19). The study protocol was approved by the Institutional Review Board of the Chungnam National University Hospital (IRB file No. 2013-01-003). This was a retrospective study, so the informed consent was waived by the board.
Statistical analysis
Clinical data were analyzed using IBM SPSS Statistics version 19, and the mean and standard deviation (SD) of each measurement were obtained. The results of clinical signs and symptoms, chest CT according to the presence or absence of eosinophilia were analyzed using the χ2 test with Fisher’s exact test. A P value <0.05 was considered as statistically significant.
Results
Clinical characteristics (Table 1)
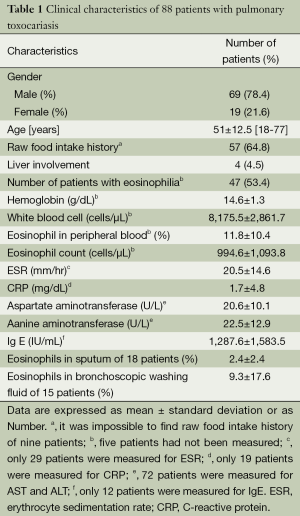
Full table
A total of 88 patients who were tested positive for antibodies against Toxocara were diagnosed with lung-involved toxocariasis. Out of 88 target patients, 69 (78%) were male and 19 (22%) were female. The mean age at diagnosis was 51 (range, 18-77) years. During the past 6 months, 57 patients (65%) had ingested uncooked cow liver, intestine, or meat; and 9 (10%) had no history of ingesting raw animal parts. Therefore, more than half of the patients had a history of eating raw animal products. Four patients (5%) were suspected of having liver abscess caused by T. canis/cati, due to the presence of hypo-dense lesions in the liver. The mean hemoglobin count was 15 g/dL, and many patients did not have accompanying anemia. The mean white blood cell count was 8,176 cells/μL (range, 4,510-18,490 cells/μL), mean eosinophil fraction in peripheral blood was 11.8% (range, 0.3-44.5%), and mean absolute eosinophil count was 995 cells/μL (range, 30-4,860 cells/μL). Mean erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were 21 mm/hr and 1.7 mg/dL, respectively. It seemed that toxocariasis was not accompanied by an increase in ESR. CRP was measured in 19 patients, and the level increased by more than 0.5 mg/dL in 3 of these patients. Therefore, it seemed that toxocariasis was not accompanied by an increase in CRP. Mean aspartate aminotransferase/alanine aminotransferase levels were 21/23 U/L, which was thought to be due to the small number of patients with liver involvement. Mean serum IgE level was 1,288 IU/mL. Eosinophil fraction in sputum and bronchial aspiration was 2.4% and 9.3%, respectively, which was higher than average. However, this was only measured in 18 and 15 patients, respectively.
Symptoms at admission (Table 2)
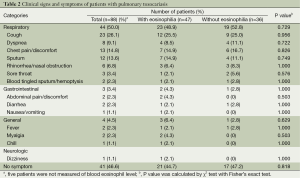
Full table
A total of 44 (50%) patients had respiratory symptoms on the day of admission, and half of the patients did not have such symptoms. Cough was the most common (26%), followed by chest pain or discomfort (15%). Gastrointestinal symptoms were seen in 3 (3%) patients, and one of these patients had abdominal discomfort, diarrhea, nausea, and vomiting. The other patients only had diarrhea or abdominal pain. Two patients (2%) had fever and 41 patients (47%) were asymptomatic. There was no statistical significance between patients with eosinophilia and those without.
Chest CT differences depending on eosinophilia (Table 3)
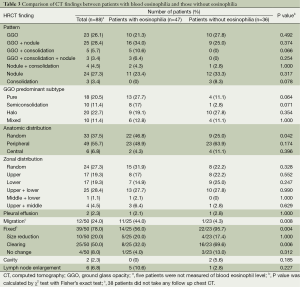
Full table
All targeted patients underwent chest CT. Among them, nodules with GGO patterns were the most common finding (25 patients; 28%), followed by nodule pattern alone (24; 27%). The pattern of GGO alone was third in group (23; 26%). In addition, two patients had cavitary lesions, and one of these patients had cavities with consolidation and the other had cavities with nodules and GGO patterns. Both of these patients did not have leukocytosis or elevation of CRP on blood test. Also, they did not have enough symptoms, such as fever on admission, to suggest of a bacterial infection. In addition, the lesions showed improvement on chest CT without any treatment, and new lesions rather appeared. Therefore, we considered these cavitary lesions to be caused by toxocariasis rather than by microbial infection. In one of the patients, decrease in the size of the cavities and thinning of the wall thickness were shown on the follow up chest CT after two weeks, even without any treatment. We started on albendazole treatment, since toxocara ELISA positivity was confirmed at that time (Figure 1). To the best of our knowledge, no previous studies have reported on cavitary lesions in pulmonary toxocariasis, and it is a novel suggestion that toxocariasis pulmonary involvement may also appear as cavitary lesions.
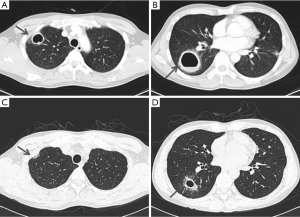
In terms of anatomical distribution, 49 patients (56%) had a peripheral distribution, 33 (38%) had a random distribution, and 6 (7%) had a central distribution. For zonal distribution, 25 patients (28%) had both upper and lower lobe involvement, 24 (27%) patients had random distribution, 17 (19%) patients had only the upper lobe involvement, and 17 (19%) patients had only the lower lobe involvement. Two patients had pleural effusion accompanied by GGO pattern in lung parenchyma, which was in the right and left lungs, respectively. Six cases had reactive enlargement of lymph nodes with infiltration of lung parenchyma.
A total of 83 patients with toxocariasis who had lung involvement underwent blood tests, and 47 (57%) had eosinophilia and 36 (43%) did not. The most common chest CT findings of patients with eosinophilia were nodules with GGO pattern which were seen in 16 patients (34%). The most common chest CT findings in patients without eosinophilia were nodules (33%). There were no significant differences in the lesion characteristics between patients with and without eosinophilia. Depending on the predominant type, patients who have GGO lesions were reanalyzed by classifying as shown in Table 3. The patients with eosinophilia relatively had many pure GGO, but those without eosinophilia had more of halo GGO. But there was no statistically significant difference.
In terms of anatomical distribution, peripheral distribution was observed in 23 (49%) patients with eosinophilia and in 23 (64%) patients without eosinophilia. However, there was no significant difference (P=0.174). Random distribution was significantly different between the two groups; patients with eosinophilia were found to be distributed more randomly (P=0.042). In 22 of 47 patients who had eosinophilia, it was difficult to assess whether the lesion was migrating or fixed, because additional chest CT was not performed within 6 months. In the remaining 25 patients, additional chest CT was performed two or three times to determine whether the lesions have improved or not. Eleven (44%) patients were identified to have migrating lesions with disappearance, improvement of existing lesions, and occurrence of new lesions elsewhere. Additional chest CTs were performed on 23 patients without eosinophilia, but only one of them had migrating lesions; there were significance differences between patients with and without eosinophilia (P=0.008). All of the lesions of these patients, except for one person without eosinophilia, were improved or fixed compared to lesions at the time of diagnosis. There was a significant difference among the lesions in patients with eosinophilia (P=0.004).
Treatment and clinical results
Twenty-seven patients diagnosed with pulmonary involvement of toxocariasis were treated with albendazole 400 mg twice daily for 5 days from the day toxocariasis was confirmed. The remaining patients did not receive any prescription. All patients showed improved symptoms within 6 months after the diagnosis regardless of albendazole treatment, and lesions decreased in size or disappeared on the last chest CT or X-ray. None of the patients had any clinical or radiological recurrence.
Discussion
Toxocariasis has become a frequent cause of eosinophilic lung disease, and its prevalence has increased. Therefore, we were interested in establishing the clinical manifestations of toxocariasis parasitic lung disease.
It is often assumed that serology, imaging, and pathological examinations are needed to diagnose toxocariasis. However, we believe that serological testing using ELISA and chest CT can be used to diagnose toxocariasis without the risk and invasiveness of biopsy (13). This type of toxocariasis serodiagnosis cross reacts with some other helminthes, such as Trichinella, Strongyloides (16), Fasciola, Ascaris, geohelminthes (20-22), and filariasis (20). Fortunately, geohelminthes have almost disappeared, filarisis has been eradicated in Korea, and it is difficult to find poly parasitism in Korea (23). Therefore, the serological test has sufficient specificity to be the best indirect test for diagnosing this infection.
In this study, the male/female T. canis infection rates were 78%/22%, which was similar to previous studies reporting 82%/12% (9) and 78%/22% (11) and are due to eating habits. Some people like to eat uncooked cow liver or stomach, as they believe raw cow liver is a “health-promoting food” (24). These foods tend to be readily consumed by men but not by women, although it has not been studied. We think that these foods that produce an aversion are consumed more frequently by men than women.
Our results are not greatly different from those of other studies on eosinophilic lung disease, which have reported random GGO patterns on chest CT (2,14). A total of 64% of our cases had lesions with GGO, so the GGO pattern is a typical radiological finding in toxocariasis with lung involvement. We found cavitary lesions, which have not been reported in other studies. We suggest that pulmonary involvement of toxocariasis may manifest as cavitary lesions.
In this study, respiratory symptoms, fever, and gastrointestinal symptoms were thought to be due to a hyper-reaction caused by T. canis larvae systemic involvement. However, 47% of the patients were asymptomatic, and most were diagnosed by the presence of an IgG antibody against Toxocara. These examinations were performed due to abnormal findings on simple X-rays during health check-ups. All patients regardless of symptoms were treated, and lesions on CT findings resolved or improved, indicating that pulmonary involvement of toxocariasis has a good prognosis. This result is similar to a previous study (9) showing that 25 of 54 patients with toxocariasis had improved symptoms and chest radiography findings without treatment. Many authors have suggested that toxocariasis is a self-healing disease; therefore, there is no need for treatment if the symptoms are mild.
A 2009 study in Korea (14) analyzed the presence of migrating masses in 58 patients with antibodies against Toxocara. The lesions resolved or improved in 48% of cases, and migrating nodules were confirmed in 35% of cases. However, they did not analyze the coexistence of eosinophilia in the patients with migrating nodules, so the relationship between these two features was not established. Peripheral blood eosinophilia is related to the degree of tissue involvement (25). One review article reported that peak eosinophil levels occur 11-30 weeks after infection, which is consistent with differences in life cycles between parasites. The spontaneous decrease in the number of eosinophils without treatment suggests active and spontaneous modulation of eosinophilia, which generally occurs when migration of the larval form ceases and the infection becomes patent (26). For this reason, we think that a pattern of migrating lesions was shown in the patients with eosinophilia in this study.
Interleukin-5 from Th2 lymphocytes triggers formation of eosinophils, which excrete cytotoxic granules and destroy parasites directly as well as necrotize tissue (27). In contrast, parasites trapped in intact cysts or in the intestinal lumen that do not invade tissues tend not to cause eosinophilia (28). Thus, the non-migrating nodules on CT in patients without eosinophilia may not be lung nodules caused by Toxocara. However, disease course of the patients improved on chest X-ray or follow-up chest CT and was similar to those with toxocariasis. Additionally, no evidence indicated another disease. Therefore, we thought that the lesions were due to the Toxocara infection.
We included patients with toxocariasis not accompanied by eosinophilia. We also described a subtype of GGO that has not been analyzed to date. However, the retrospective nature of the study design and the lack of follow-up CT are limitations of this study. Future studies involving more patients with follow-up chest CT and more clinical and radiological characteristics should be performed for a more accurate investigation of pulmonary involvement of toxocariasis.
Conclusions
In conclusion, not all of the patients with toxocariasis had eosinophilia, and eosinophilia seemed to be related to lesion migration. The lesions of lung involved toxocariasis with eosinophilia tend to be more migrating, and those without eosinophilia tend to be fixed.
Acknowledgements
Authors’ contributions: Jeong Eun Lee designed the overall study with contributions from Sun Young Kim, Ju Ock Kim, Sung Soo Jung, Hee Sun Park. Bo Mi Park and Sang Ok Jeong collected and analyzed data. Bo Mi Park wrote the paper.
Disclosure: The authors declare no conflict of interest.
References
- Hong ST. Gungnae gisaengchung gamnyeomjeungui hyeonhwang. J Korean Med Assoc 1998;41:737-45.
- Shin S. Parasitic zoonosis transmitted by pet animals. J Korean Med Assoc 2004;47:527-34.
- Yang HJ, Yoon YB, Yi H, et al. Prevalence of Intestinal Parasites Infection of Dogs in Chonbuk Province. Korean J Vet Serv 1992;15:7-16.
- Habluetzel A, Traldi G, Ruggieri S, et al. An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet Parasitol 2003;113:243-52. [PubMed]
- Anaruma Filho F, Chieffi PP, Correa CR, et al. Human toxocariasis: a seroepidemiological survey in the municipality of Campinas (SP), Brazil. Rev Inst Med Trop Sao Paulo 2002;44:303-7. [PubMed]
- Richards DT, Harris S, Lewis JW. Epidemiology of Toxocara canis in red foxes (Vulpes vulpes) from urban areas of Bristol. Parasitology 1993;107:167-73. [PubMed]
- O’Lorcain P. Prevalence of Toxocara canis ova in public playgrounds in the Dublin area of Ireland. J Helminthol 1994;68:237-41. [PubMed]
- Barriga OO. A critical look at the importance, prevalence and control of toxocariasis and the possibilities of immunological control. Vet Parasitol 1988;29:195-234. [PubMed]
- Kwon NH, Oh MJ, Lee SP, et al. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol 2006;85:233-8. [PubMed]
- Kim YJ, Kyung SY, An CH, et al. The characteristics of eosinophilc lung diseases cause by Toxocara canis larval infestation. Tuberc Respir Dis 2007;62:19-26.
- Vijayan VK. Tropical parasitic lung diseases. Indian J Chest Dis Allied Sci 2008;50:49-66. [PubMed]
- Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 2003;16:265-72. [PubMed]
- Yoon YS, Lee CH, Kang YA, et al. Impact of toxocariasis in patients with unexplained patchy pulmonary infiltrate in Korea. J Korean Med Sci 2009;24:40-5. [PubMed]
- van Assendelft OW. Reference values for the total and differential leukocyte count. Blood Cells 1985;11:77-96. [PubMed]
- Jacquier P, Gottstein B, Stingelin Y, et al. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol 1991;29:1831-5. [PubMed]
- Cheon JE, Lee KS, Jung GS, et al. Acute eosinophilic pneumonia: radiographic and CT findings in six patients. AJR Am J Roentgenol 1996;167:1195-9. [PubMed]
- Johkoh T, Müller NL, Akira M, et al. Eosinophilic lung diseases: diagnostic accuracy of thin-section CT in 111 patients. Radiology 2000;216:773-80. [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [PubMed]
- Mohamad S, Azmi NC, Noordin R. Development and evaluation of a sensitive and specific assay for diagnosis of human toxocariasis by use of three recombinant antigens (TES-26, TES-30USM, and TES-120). J Clin Microbiol 2009;47:1712-7. [PubMed]
- Magnaval JF, Fabre R, Maurières P, et al. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res 1991;77:697-702. [PubMed]
- Romasanta A, Romero JL, Arias M, et al. Diagnosis of parasitic zoonoses by immunoenzymatic assays--analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis, and Ascaris suum. Immunol Invest 2003;32:131-42. [PubMed]
- Jin Y, Shen C, Huh S, et al. Serodiagnosis of toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol 2013;51:433-9. [PubMed]
- Lim JH. Foodborne eosinophilia due to visceral larva migrans: a disease abandoned. J Korean Med Sci 2012;27:1-2. [PubMed]
- John TJ, Walker DH. Tropical infectious diseases, principles, pathogens, and practice. In: Guerrant RL, Walker DH, Weller PF. eds. Philadelphia: Churchill Livingstone, 1999:1123-32.
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 2004;113:30-7. [PubMed]
- Rothenberg ME. Eosinophilia. N Engl J Med 1998;338:1592-600. [PubMed]
- Leder K, Weller PF. Eosinophilia and helminthic infections. Baillieres Best Pract Res Clin Haematol 2000;13:301-17. [PubMed]


