
Coexisting active pulmonary tuberculosis in tuberculous spondylitis: the prevalence and the role of chest CT
In clinical practice, tuberculous (TB) spondylitis alone does not warrant patient isolation (1). We experienced patients with underlying TB spondylitis involved the appearance of only subtle lung lesions on the chest radiograph, but chest computed tomography (CT) scans showed active pulmonary TB. If the coexisting lung lesion is present, it will be helpful to early diagnosis and treatment of TB. However, a coexisting lung lesion is easy to overlook, particularly on plain chest radiography. And there is a lack of clear evidence that TB spondylitis will require additional chest CT scans in previous studies. Therefore, we hypothesized that pre-diagnostic consideration of the coexistence of an active lung lesion in patients with suspicious TB spondylitis can help diagnose TB.
We retrospectively reviewed consecutive 50 patients with a histological or microbiologically confirmed diagnosis of TB spondylitis among surgically confirmed TB spondylitis between January 2005 and December 2015 (IRB No. KHU 2017-08-030). Patients’ demographic information and medical history were reviewed using clinical charts. Two radiologists (So Youn Shin and Eun Jung Shim) retrospectively reviewed all images to reach consensus. We analyzed spine CT scans and magnetic resonance (MR) images for each affected level and also reviewed chest plain radiographs and CTs to evaluate lung involvement in TB. We defined active pulmonary TB as follows: (I) centrilobular nodules, (II) branching linear opacities with nodularity (tree-in-bud sign), and (III) lobular or patchy lesions of consolidation and cavitation (2). The presence of miliary TB and pleural effusion were also analyzed. We also reviewed microbiological analyses to determine the level of activity of TB. Statistical analysis was performed to investigate the presence of concomitant active pulmonary TB in TB spondylitis patients according to the level of TB spondylitis using SPSS 23 (Statistical Package for Social Science, version 23.0, IBM Corporation, Chicago, IL, USA) and R 3.5.1 (http://cran.r-project.org), with P values below 0.05 considered statistically significant.
Among 50 patients with surgically confirmed TB spondylitis, there was no significant difference in gender (male: female =24:26) and the mean age was 51.82±18.79 years old (range, 15–79 years). Only nine (18%) had a history of pulmonary TB. Table 1 shows the results of radiologic image analysis. The most frequently involved region of the spine was lower T (T7–12) regions, and the most frequently affected region was T12. Twenty-one (42%) showed concomitant active pulmonary TB on radiologic image analysis. In microbiologic results, of the 21 patients with TB spondylitis who had concomitant active pulmonary TB on radiologic image analysis, only one (4.76%) tested positive in a sputum AFB and was therefore regarded as potentially contagious at initial clinical diagnosis. In statistical analysis, TB spondylitis involving the upper (C or T) spinal region had a significant correlation with coexisting active pulmonary TB (P=0.0033), compared with lower spine involvement (Table 2).
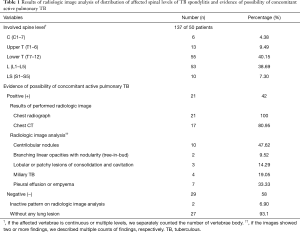
Full table
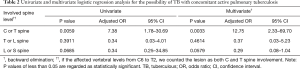
Full table
According to several studies of affected levels of TB spondylitis, lower thoracic and upper lumbar regions are the most commonly affected sites (3-5). In our study, consistent with previous studies, the lower T (T7–12) regions were the most commonly involved sites.
It is known that the probability of concomitant pulmonary TB in TB spondylitis patient shows wide variation among countries (4,6). In an article of literature review by Schirmer et al. (4), the probability of concomitant pulmonary TB in TB spondylitis patients varies from 8% to 100%. In recent large scale studies for spinal TB, the incidences of concomitant pulmonary TB show from 14.37% to 28% (7-9). The lower rates of concomitant pulmonary TB in those studies compared to our study (42%) may be related to differences in diagnosis of ‘active pulmonary TB’—clinical versus imaging assessment. In clinical practice, sputum acid-fast bacilli (AFB), culture, or TB polymerase chain reaction (PCR) are not sensitive enough for screening for active pulmonary TB. We demonstrated that TB spondylitis involving the upper (C or T) spinal regions was significantly correlated with a coexisting active pulmonary TB. Therefore, we suggest that a patient with TB spondylitis involving the upper (C or T) spinal region would need to be assessed the concomitant active pulmonary TB. Moreover, because patients with TB spondylitis often complain of back pain and this condition can make it difficult to undergo a posteroanterior chest radiograph in the erect position and therefore to delineate lung nodules on a spine CT, we want to emphasize that radiologic imaging could be an additional approach for diagnosis of TB to avoid missing cases of potentially active pulmonary TB.
A negative AFB smear is commonly regarded as having a low infectivity and is common at initial diagnosis, which make it difficult to diagnosis and treat the disease early. However, respiratory transmission could also occur (17%) from person with sputum smear-negative TB (10,11). In our study, among concomitant active pulmonary TB on radiologic image analysis, only one patient (4.76%) tested positive in sputum AFB test and three patients (14.29%) showed positive in sputum TB PCR and regarded as potentially contagious at initial clinical approach. Chest radiographs are not specific and it can appear normal even when the disease is present (12). Neither military tuberculosis nor pleural effusion is usually not considered as infectious, however, these findings may help in the diagnosis of spinal TB. This suggests greater attention should be paid to the potential for TB transmission despite negative smear results.
In conclusion, we found 42% of TB spondylitis had a coexisting potentially active pulmonary TB lesion on radiologic image analysis. This literature showed the higher co-morbidity of active pulmonary TB and TB spondylitis, the higher potential risk of nosocomial infection of TB. Because the possibility of concomitant active pulmonary tuberculosis in TB spondylitis patient can be easily overlooked, there is a chance of the possibility of nosocomial infection of TB. And we recommend that chest CT (at least low-dose chest CT) would be useful in the initial evaluation of TB spondylitis, especially with upper (C or T) spinal region involvement, in spite of subtle evidence of active pulmonary TB on plain chest radiography. It would be helpful in diagnosing TB earlier and preventing airborne dissemination.
Acknowledgments
I would like to thank all the staffs and participants of 2016 KSR CRMC advanced course for guiding and supporting in writing of this article. I would also like to extend my thanks to Su Jin Jeong, a statist at Kyung Hee University Medical Center for supporting statistical analysis of this article. This work was presented in part at the World Congress of Thoracic Imaging (WCTI) 2017, Boston, MA, USA.
Funding: This research was supported by a grant from Kyung Hee University (KHU-20160544).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.48). SHL serves as an unpaid editorial board member of Journal of Thoracic Disease from Apr 2019 to Mar 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glaziou P, Sismanidis C, Floyd K, et al. Global epidemiology of tuberculosis. Cold Spring Harb Perspect Med 2014;5:a017798. [Crossref] [PubMed]
- Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834-44. [Crossref] [PubMed]
- Burrill J, Williams CJ, Bain G, et al. Tuberculosis: a radiologic review. Radiographics 2007;27:1255-73. [Crossref] [PubMed]
- Schirmer P, Renault CA, Holodniy M. Is spinal tuberculosis contagious? Int J Infect Dis 2010;14:e659-66. [Crossref] [PubMed]
- Ramírez-Lapausa M, Menéndez-Saldaña A, Noguerado-Asensio A. Extrapulmonary tuberculosis. Rev Esp Sanid Penit 2015;17:3-11. [Crossref] [PubMed]
- Leibert E, Schluger NW, Bonk S, et al. Spinal tuberculosis in patients with human immunodeficiency virus infection: clinical presentation, therapy and outcome. Tuber Lung Dis 1996;77:329-34. [Crossref] [PubMed]
- Chung SM, Kim NH, Kim YA, et al. Clinical studies of tuberculosis of the spine. Yonsei Med J 1978;19:96-104. [Crossref] [PubMed]
- Wang H, Li C, Wang J, et al. Characteristics of patients with spinal tuberculosis: seven-year experience of a teaching hospital in Southwest China. Int Orthop 2012;36:1429-34. [Crossref] [PubMed]
- Shi T, Zhang Z, Dai F, et al. Retrospective Study of 967 Patients With Spinal Tuberculosis. Orthopedics 2016;39:e838-43. [Crossref] [PubMed]
- Hernandez-Garduno E, Cook V, Kunimoto D, et al. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax 2004;59:286-90. [Crossref] [PubMed]
- Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999;353:444-9. [Crossref] [PubMed]
- Ryu YJ. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis (Seoul) 2015;78:64-71. [Crossref] [PubMed]


