
Decreased ventilatory efficiency during incremental exercise in bronchiectasis
Bronchiectasis is characterized by aberrant bronchial dilatation (1). The structural changes have been associated with ventilatory dysfunction, exercise intolerance and ventilatory inhomogeneity (2,3) that may aggravate during exercise. A decreased ventilatory efficiency, reflected by the high carbon dioxide ventilatory equivalent (VE/VCO2), has been identified during incremental exercise in patients with COPD, especially when complicated with heart failure (4). At earlier stages of bronchiectasis, exercise intolerance might have become evident before cardiovascular complications developed (5). The mechanisms underlying exercise intolerance in patients with bronchiectasis without heart failure are, however, not entirely clear. By using incremental cycle ergometer, we determined the ventilation-gas exchange abnormalities in patients with bronchiectasis not complicated with physician-diagnosed heart failure.
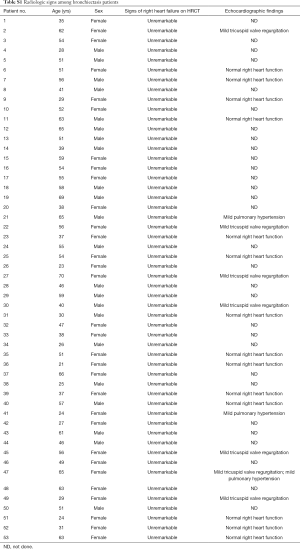
Participants were enrolled between March 2017 and December 2018. The diagnosis of bronchiectasis was confirmed according to clinical symptoms (including chronic cough and sputum production) and chest high-resolution computed tomography within 12 months. For eligibility of study entry, patients with bronchiectasis were aged 18 years or greater, remained clinically stable, had no antibiotics use for >4 weeks, and had no physician-diagnosed heart failure. Patients with malignancy, heart attack within 6 months, or acute upper respiratory tract infections within 4 weeks were excluded. We also screened for possible right heart failure based on the radiologic signs on chest high-resolution computed tomography and echocardiography (among 23 out of 53 patients who have undergone the measurement). Healthy subjects were aged 18 years or greater, had no significant diseases influencing on exercise testing. Our study was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University. All participants gave written informed consent.
Patients underwent severity assessment with bronchiectasis severity index, impulse oscillometry, multiple-breath nitrogen washout test (which also measured the lung clearance index), spirometry, and cardiopulmonary exercise testing (CPET). All measurements were made with the commercial lung function testing instrument (Jaeger MasterScreenTM. Carefusion Co., Ltd.). The methods for determining the etiology of bronchiectasis have been described previously (2). Healthy subjects underwent spirometry and CPET only. A cycle ergometer equipped with real-time gas-analyzer (COSMED Inc., Italy) was applied for CPET, including steady-state rest, 20-W increase in work rate at 1-min intervals until symptom limitation, and recovery. Main parameters comprised breath-by-breath ventilatory volume (VE), tidal volume (VT), oxygen consumption (VO2), carbon dioxide production (VCO2), oxygen pulse (HR/VO2), oxygen ventilatory equivalent (VE/VO2), VE/VCO2, and end-tidal carbon dioxide partial pressure (PetCO2). Fatigue and dyspnea were graded using a 10-point scale (Borg’s scale) at rest and after maximal exercise. We took reference on a published study to define the cut-off value of hypocapnia based on the partial pressure of end-tidal carbon dioxide (PetCO2) at different time points of exercise [4].
Statistical analysis was performed using Graphpad Prism 5.0 (Graphpad Inc., USA). Kolmogorov-Smirnov test was used to assess normality of the continuous variables. Independent t-test or Mann-Whitney test was conducted for two-group comparisons, and multiple-group comparisons (among patients with or without exercise-induced hypocapnia and healthy controls, see the definition in the results section below) were conducted with one-way analysis-of-variance or Kruskal-Wallis test, followed by Bonferroni correction. Correlation analysis was done using Spearman’s test.
Seventy-nine participants underwent screening, of whom 53 patients with bronchiectasis and 16 controls were analyzed. Reasons for the exclusion of bronchiectasis patients included joint deformity (n=2), FEV1 <30% predicted (n=5), recent trauma (n=2) and heart failure (n=1). Fifty-eight point five percent of participants were middle-aged females who never smoked. The baseline characteristics are displayed in Table 1. Bronchiectasis severity was mostly graded as mild-to-moderate. Idiopathic (45.2%) and post-infection (35.8%) constituted the most common etiologies. None of the eligible participants had documented cardiovascular disorders except for grade II hypertension in one bronchiectasis patient. Sixty-seven-point nine percent of the bronchiectasis patients had restrictive/obstructive/mixed ventilatory dysfunction, and the mean FEV1 was 72% predicted among 53 patients. Twenty-one (40%) patients with bronchiectasis had an increased ratio of residual volume to total lung capacity. None of the patients with bronchiectasis had radiologic signs of clinically evident heart failure. Three patients had mild pulmonary hypertension (Table S1). Table S2 demonstrates the lung function characteristics of patients with bronchiectasis and healthy controls at rest. Overall, bronchiectasis patients yielded significantly lower levels of FVC and FEV1 predicted% than healthy controls (both P<0.05).
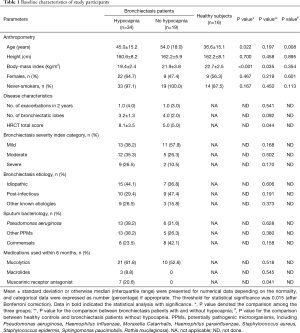
Full table
Full table
Full table
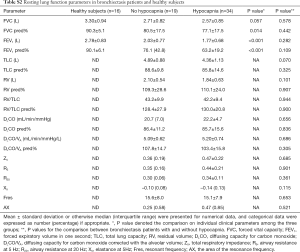
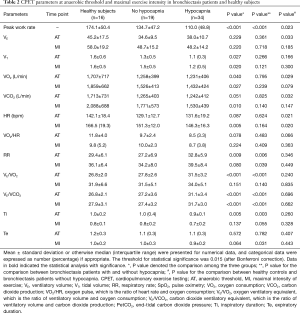
VE/VCO2 was significantly higher in patients with bronchiectasis than in healthy controls throughout exercise (all P<0.01). However, there was no remarkable difference in VE/VCO2 between healthy controls and bronchiectasis patients without hypocapnia at anaerobic threshold or maximal intensity exercise. VE/VCO2 correlated strongly with PetCO2 at maximal exercise (Figure 1), but not resting residual volumes or bronchiectasis severity. VE/VCO2 nadir correlated significantly with the simultaneously measured PetCO2 (P<0.01). We next stratified bronchiectasis patients based on PetCO2 because it explained substantially for exertional ventilatory responses. Patients with consistently low PetCO2 throughout exercise (<35 mmHg, hypocapnia group) did not differ from their counterparts regarding resting ventilatory function, diffusing capacity, lung volumes and airway resistance (all P>0.05, Table S2) (Table 1).
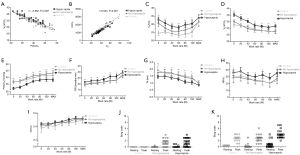
Patients with hypocapnia yielded markedly lower peak work rate and shorter inspiratory time, but higher VE/VO2 and VE/VCO2, at anaerobic threshold than patients without (all P<0.05). At submaximal exercise, patients with hypocapnia yielded systematically higher VE, VE/VO2 and VE/VCO2 but lower PetCO2 than those without (all P<0.05, Table 2). Higher VE was associated with consistently higher respiratory rate and shorter expiratory time at different time points of exercise in patients with hypocapnia as compared with those without hypocapnia. The higher VE was, however, not associated with significant differences in VT, the ratio of respiratory rate to inspiratory time, and the ratio of inspiratory to total respiratory time between patients with and without hypocapnia (Figure 1). Moreover, the disease severity (i.e., bronchiectasis severity index) did not correlate with most of the lung function parameters at different time points during exercise (data not shown).
Full table
There was no substantial difference in dyspnea or fatigue ratings at baseline between patients with and without hypocapnia. At maximal exercise, the increase was similar for dyspnea ratings although appearing greater for fatigue ratings in patients with hypocapnia.
Efficient ventilation is crucial to the exercise tolerability. In adults with cystic fibrosis, the ventilatory efficiency correlated significantly with the imaging severity of bronchiectasis that affects ventilatory inhomogeneity (6). First, our study has reaffirmed the ventilatory inefficiency (high VE/VCO2 levels) throughout incremental exercise in bronchiectasis. The high VE/VCO2 has previously been attributed to excessive ventilation in COPD-heart failure overlap (4), but the mechanisms in patients without clinically overt heart failure are less clear. The VE/VCO2 was recorded at fixed work rate but might get close to the VE/VCO2 nadir at anaerobic threshold or maximal exercise. By stratification based on PetCO2 (which mirrored arterial or capillary carbon dioxide partial pressure), we have identified patients with hypocapnia at different time points during exercise. Because PetCO2 might not invariably be a reliable surrogate of PaCO2, caution should be exercised in interpreting some of our findings. The reduced exercise tolerance might have also resulted from the dead space ventilation (7), because airflow limitation (47% among 53 patients) and increased residual volumes (~30.2%) were present at resting.
Second, patients with hypocapnia had more prominent airflow limitation, and consistently higher respiratory rate and a trend towards shorter expiratory time during exercise. Hence, the inspiratory constraint could also be due to the greater VE. In COPD, although the high VE/VCO2 could be partially compensated by the inspiratory constraint and hypercapnia, VE/VCO2 may remain high because of the exaggerated dead space ventilation and greater respiratory drive, particularly when complicated with heart failure (4). We cannot preclude subclinical pulmonary hypertension or heart failure, particularly in those with bilateral bronchiectasis (8). This might help partially explain for the lack of significant difference in dyspnea scale between patients with and without hypocapnia.
Despite efforts to identify possible signs of right heart failure, echocardiography was not performed in every individual, and therefore we might have included some patients with early-stage right heart insufficiency. Catheterization was not performed before incremental exercise testing, thus pulmonary hypertension which is a crucial indicator of right heart failure could have been under-diagnosed. However, the invasiveness and requirement of medical expertise have limited the applicability of catheterization as a routine measurement in our clinical setting.
In summary, the greater ventilatory demand might have contributed to inspiratory constraint, partly explaining for the decreased ventilatory efficiency on exertion in patients with bronchiectasis without physician-diagnosed heart failure. Further studies are needed to determine whether interventions (i.e., pulmonary rehabilitation) could help improve the outcomes of bronchiectasis by increasing the ventilatory efficiency.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation No. 81870003, Pearl River S&T Nova Program of Guangzhou No. 201710010097 and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2017 (to Prof. Guan), The Impact and Mechanisms of Physical, Chemical and Biological Interventions on the Development and Outcome of Acute Lung Injury No. 81490534, National Key Technology R&D Program No. 2018YFC1311902, Guangdong Science and Technology Foundation No. 2019B030316028 (to Prof. Zhong).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.113). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017. [Crossref] [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS One 2014;9:e113373. [Crossref] [PubMed]
- Ramsey KA, Rosenow T, Turkovic L, et al. Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016;193:60-7. [Crossref] [PubMed]
- Rocha A, Arbex FF, Sperandio PA, et al. Excess ventilation in chronic obstructive pulmonary disease-heart failure overlap. implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 2017;196:1264-74. [Crossref] [PubMed]
- Hena R, Alaparthi GK, Shyam Krishnan K, et al. Cardiorespiratory responses to glittre ADL test in bronchiectasis: a cross-sectional study. Can Respir J. 2018;2018:7470387. [Crossref] [PubMed]
- Crisafulli E, Teopompi E, Luceri S, et al. The value of high-resolution computed tomography (HRCT) to determine exercise ventilatory inefficiency and dynamic hyperinflation in adult patients with cystic fibrosis. Respir Res 2019;20:78. [Crossref] [PubMed]
- Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 2015;191:1384-94. [Crossref] [PubMed]
- Wang L, Jiang S, Shi J, et al. Clinical characteristics of pulmonary hypertension in bronchiectasis. Front Med 2016;10:336-44. [Crossref] [PubMed]


