
Suggested management of e-cigarette or vaping product use associated lung injury (EVALI)
Respiratory care physicians are confronted with a new pulmonary syndrome linked to e-cigarette consumption. Electronic cigarettes have been known since 2003 and are known by different names such as “e-cigs”, “vape pens”, “e-hookahs” or “electronic nicotine delivery systems (ENDS)”. In recent years, e-cigarettes have advanced from the more primitive first-generation devices to the current fourth generation devices. The modern devices feature higher voltages and higher temperatures and therefore produce more aerosol volume from heating liquids. Inhaling these aerosolized liquids is also called vaping. Although the liquid composition has many possible variations, it mostly contains propylene glycol (PG), glycerin, flavorings with or without nicotine. E-cigarettes from JUUL, which is the top-selling brand in the United States (US), have a USB-like shape and all contain nicotine in high doses. These high nicotine levels are achieved by using specific nicotine salts that allow inhalation of such high nicotine doses with less irritation than with traditional tobacco cigarettes. Other devices have also been used to inhale a number of psychoactive drugs added to e-liquids, such as the cannabis derivative tetrahydrocannabinol (THC), cannabinoid oils (CBD), cocaine, heroin and amphetamines (1).
Unfortunately, the higher temperatures of the newer e-cigarette devices, not only led to higher volume aerosols, but also produce more by-products, such as heavy metals including nickel, tin and lead, and also volatile organic compounds. Moreover, the aerosol particles can lead to the formation of other compounds by interaction of the particles themselves, resulting in products such as acetone, formaldehyde, nitrosonornicotine and tobacco-specific nitrosamine. Initially, e-cigarettes were promoted as a way of stopping or reducing traditional tobacco smoking. However, there have been concerns about early nicotine addiction by youths experimenting with e-cigarettes and recent reports of a new pulmonary syndrome with a wide spectrum of lung pathology, which is related to e-cigarette use (2). In some patients, this syndrome called e-cigarette or vaping product use associated lung injury (EVALI), has led to severe respiratory failure, or even death. As of February 4th, 2020, a total of 2,758 hospitalized EVALI cases have been reported to the Centers of Disease Control and Prevention (CDC) in the US and 64 deaths have been reported (www.CDC.gov). For physicians confronted with patients with this new pulmonary syndrome called EVALI, sometimes also reported as “vaping associated lung injury” (VAPI), the diagnosis and optimal management present a real challenge. Until now, no single causative agent has been identified that clearly is responsible for EVALI. However, current evidence suggests that vitamin E acetate (VEA) and THC may play a major role in the development of EVALI (3). Other components of the vaporizing devices and liquids have been implicated to play a role in the pathogenesis of EVALI, especially liquids from the illegal markets and self-mixed liquids (4). As more research emerges, the picture of the main causative agents is likely to become more definitive, whereby it is expected to be not a single component but more likely combinations of various components that increase the chances of manifesting clinically relevant symptoms of EVALI.
In this article, we summarize the treatment strategies described in the literature and propose a flow chart as a guide to clinicians, based on the current literature. Between 2017 and January 2020 in PubMed we found 15 case reports describing the treatment of EVALI patients (Table 1). There were also seven case series on EVALI patients, however, in case series the treatments were only described in general for the whole group of patients. So far, there are no randomized controlled trials that have evaluated therapy in EVALI (4-18). Therapy therefore remains largely empiric or symptom-guided. The authors were involved in the treatment of such a case (4).
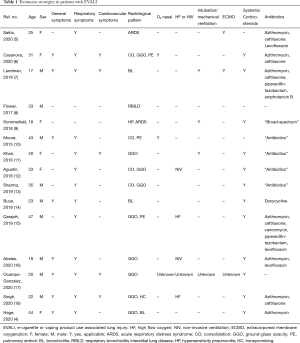
Full table
What is EVALI?
The diagnosis of EVALI is mainly a diagnosis of exclusion of other diseases (1). This can be difficult in the influenza season, as there is no single clinical, biochemical or radiological finding that is specific for EVALI. Furthermore, EVALI can be associated with other viral or bacterial infections as well. Patients mostly present with respiratory symptoms such as cough, thoracic pain, dyspnea or hemoptysis (19). In addition, combinations with gastrointestinal symptoms including abdominal pain, nausea, vomiting and diarrhea are possible. Isolated gastrointestinal symptoms are rare. Constitutional symptoms are frequent, such as weakness, myalgias, night sweats, fatigue, malaise, chills, weight loss, diaphoresis and headache (2,7,10,19-25). Symptoms are unspecific so other diagnoses may have been made previously: in a case series of 12 patients, 58% of patients with EVALI had a previous health-care visit and 17% received previous antibiotic therapy (22), and as such might have been misdiagnosed previously. Other non-specific symptoms have been reported, such as sore throat, nasal congestion, headache, epistaxis, odynophagia, leg pain and back pain (22). History taking should include questions about the vaping product (both liquids and device), vaping frequency and intensity and concurrent smoking of traditional tobacco cigarettes. There seems to be a higher risk in vaping THC oils and VEA (26). In THC oils, both VEA and vitamin-A (retinoic acid) are used to dissolve and dilute the oils along with mineral coconut oil and triglyceride medium chain oil (27). In other, non-THC, e-liquids, VEA is also used as a thickening agent (27). Blount et al. (28) demonstrated VEA, coconut oil and limonene (terpene) in 94%, 2%, and 3% of bronchoalveolar lavage (BAL) fluid of patients with EVALI patients, respectively. VEA has been shown to act as a carrier for drug delivery and as such could serve as a carrier for THC in the blood and brain of users (27). Interaction with phospholipids and surfactants of the epithelial lining fluid, due to its oxidant/radical derivatives, may be a possible mechanism of VEA toxicity in vaping (27,29,30). Although e-cigarette flavors could be another causative agent, there are no reports on other potentially causative ingredients besides VEA (27).
In patients with EVALI, the radiographic findings, which are frequently encountered, isolated or combined, include centrilobular ground-glass nodules, bilateral confluent ground-glass opacities (GGO), with frequent subpleural sparing and crazy-paving (31). In the literature, these GGO have basilar and dependent predominance, but exceptions with upper lobe and anti-dependent predominance have been described (32,33). Additionally, small pleural or pericardial effusions, enlarged hilar or mediastinal lymph nodes and mild bronchial wall thickening have been described (32).
EVALI presents radiologically as an interstitial pneumonia, of which ten different types have been described in literature (8-12,32,34-38). If one of these patterns is detected, one should always specifically ask patients about e-cigarette consumption, as cessation of e-cigarette consumption is a very important step in management of EVALI (19).
Interstitial disease patterns
The underlying interstitial disease patterns are described in Table 2 (4,8-12,34-36,38). Henry et al. (38) and Thakrar et al. (31) wrote very useful articles reviewing the different possible interstitial lung injuries in patients with EVALI.
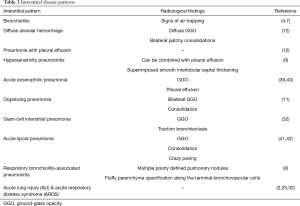
Full table
Which additional investigations are necessary?
The physical examination is rather unspecific (9-11,20,23). Temperature, vital signs and pulse oximetry are important assessments (Figure 1). Patients can present with fever, tachypnea, tachycardia, hypoxemia (SpO2 <95% breathing room air). Respiratory symptoms can be cough, thoracic pain, dyspnea and hemoptysis. Gastro-intestinal complaints are mentioned either as only organ system affected (rarely) or in combination with respiratory symptoms. These symptoms can include abdominal pain, nausea, vomiting and diarrhea. Many patients with EVALI have constitutional symptoms as well, presenting with general weakness, myalgia, night sweats, fatigue, malaise, chills, weight loss, diaphoresis and headache (22). Lung auscultation can be normal or reveal crackles and wheezing. Signs of bronchiolitis (inspiratory squeaks) should not be missed since this may influence choice of treatment (see below) (4). Laboratory investigations will show elevated infection parameters such as elevated C-reactive protein (CRP), procalcitonin and neutrophilia (26).

In addition, liver transaminases (aspartate aminotransferase and alanine aminotransferase) should be determined, as these are elevated in 50% of patients with EVALI (26).
Arterial blood gas analysis is advisable, since EVALI patients may deteriorate very rapidly. To exclude other diseases, blood cultures, sputum culture and Gram staining, urine Legionella and Pneumococcus antigen should be considered. A urine drug screen should be performed to search for drugs, as patients may hesitate to report drug use by inhalation using their e-cigarettes. Depending on the clinical situation of the patient, a spirometry and CO-diffusion capacity measurement may be considered. Depending on the season, a nasal and throat swab for a respiratory multiplex PCR should include testing for influenza virus. Radiologic examination should at least include a chest X-ray, but preferentially a chest CT-scan. In all patients with an abnormal chest X-ray, a chest CT-scan is indicated. In case of radiologic abnormalities, a bronchoscopy with BAL can guide further management, to exclude bacterial and/or viral co-infections in patients with EVALI. In clinically instable patients a bronchoscopy with BAL can be considered after intubation. Beside the microbiological analysis of the BAL, the pathological analysis could include the Oil Red O or Sudan black staining to search for lipid-laden macrophages, as these have been described in a number of patients with EVALI (2). The clinical relevance of lipid-laden macrophages however is debated (17,43). In patients in whom a Diffuse Alveolar Hemorrhage is suspected, a Prussian blue iron staining should be performed (12).
As EVALI is a diagnosis of exclusion, patients have also undergone surgical wedge resections to exclude important other diseases (8).
How should we treat EVALI?
First of all, patients should be advised to stop vaping or dabbing (and ideally also stop smoking traditional tobacco cigarettes) immediately (19). Secondly, consulting an intensivist should be considered, because patients with EVALI can show a sudden rapid and dramatic clinical deterioration within one or two days, needing high flow oxygen therapy, (non)-invasive ventilation, or even extracorporeal membrane oxygenation (ECMO) (19). In patients with EVALI, almost 50% will be admitted to an intensive care unit, of whom another 50% will need mechanical ventilation (19). Pharmacological treatment is currently not supported by systematic clinical studies, but case reports and case series are available. Most patients with EVALI improve on high doses of systemic corticosteroid treatment (e.g., initially intravenous methylprednisolone, followed by 1 mg/kg prednisolone daily). One case series reported that 82% of patients improve due to corticosteroids (19). However, the natural course without corticosteroids is unknown. In case of suspected primary infectious disease, corticosteroids could be withheld, for example in patients with fungal pneumonia. In case of co-infection with influenza this will present a dilemma, as corticosteroid treatment in influenza is associated with increased mortality and hospital-acquired infection, although the evidence relates mainly to high-dose corticosteroid treatment and the quality of evidence is low (44). Antibiotic and/or antiviral treatment should also be considered in EVALI patients, as EVALI can be associated with bacterial or viral pneumonia. The cause of this association could be the alteration of the innate immunity and airway cytokines, which can be modified by e-cigarette inhalation, increasing the virulence of colonizing bacteria (45). Cytotoxicity has been demonstrated to airway cells in vitro, after acute exposure to e-cigarette vapor, and also has been shown to decrease the function of macrophages and neutrophil antimicrobial function (45). Moreover, inhalation of e-cigarette vapor leads to increased markers of inflammation in BAL and serum (45). The initial antibiotic treatment should be dependent on the local or national antibiotic guidelines, in the current medical literature only empiric treatments have been mentioned. Most patients received combined ceftriaxone and azithromycin (Table 1). However, in case series, alternative treatments, predominantly given in combination with other antibiotics, were vancomycin, piperacillin-tazobactam and levofloxacin. Interestingly, it has been postulated that vaping increases susceptibility to pneumococcal infection by increasing oxidative stress due to the redox-active metals in the vapor, leading to increased platelet-activating factor receptor (PAFR) expression (46). Unfortunately, it is currently unknown whether pneumococcal infections are the main co-infection in EVALI patients, and whether pneumococcal vaccination would be indicated in vaping patients. Pneumococcal adhesion to airway cells was attenuated by the antioxidant N-acetyl cysteine in vitro, but clinical data in EVALI patients from case reports and case series supporting the use of N-acetyl cysteine is not available (46). Vaping has been shown to increase the risk of thrombogenesis in mice. So far there are no case reports showing pulmonary embolism or deep venous thrombosis in EVALI patients (47). Therefore, routine therapeutic anticoagulation is not indicated.
Patients with suspected influenza should be treated with prompt antiviral treatment, especially in case of severe or progressive illness, high risk for influenza complications, and in hospitalized patients (CDC recommendation).
In EVALI patients with clinical and/or radiological suspicion of bronchiolitis, the therapy should include a macrolide based on its immunomodulatory effects. In case of bronchospasm, nebulized ipratropium and salbutamol or albuterol should be considered as part of the symptomatic treatment to improve the clinical situation (4,10).
Although EVALI can present with severe signs and symptoms, some patients might be treated as outpatients initially. According to the CDC, patients with normal oxygen saturation (SpO2 ≥95%), without respiratory distress or comorbidities that might compromise pulmonary function, could be treated as out-patients, if they have reliable access to health care and have strong social support systems. A follow-up in these outpatients within 24–48 hours after initial evaluation is mandatory (26).
Patients with preexisting lung and/or cardiovascular disease and in case of pregnancy hospitalization should be considered. In older patients, the risk of respiratory failure leading to intubation with mechanical ventilation is higher. In the registry of the CDC, a total of 2,409 patients with EVALI had been documented by November 2019 of which 95% required hospitalization (48). The increased awareness among physicians will probably increase the number of patients with EVALI, and probably also more patients with relatively mild disease may be detected. Diagnosis of EVALI in patients with mild disease may have been previously easily missed and/or misdiagnosed due to the novelty of this diagnosis. The CDC recently changed the inclusion criteria for the registry from including all patients with EVALI to patients requiring hospitalization and those leading to death due to EVALI.
Pathogenetical mechanisms possibly influencing treatment
After exposure to e-cigarette vapor, in cultured lung cells, one in vitro study showed changes in bacterial phenotype associated with increased virulence (49).
In animal experimental studies, e-cigarettes lead to increased production of proinflammatory cytokines, increasing the risk of bacterial (S. pneumoniae) or viral (H1N1 influenza) infection (50-52). This finding would favor the use of antibiotic treatment, including at least a beta-lactam or cephalosporine, as well as antiviral treatment if symptoms are suggestive of viral infection.
Two possible causative agents leading to immunosuppression linked to e-cigarette use are PG and glycerol (GLY) (53). These substances are used because of their hygroscopic properties for flavors in e-liquids, and may have a direct and indirect effect on the airways. Direct effect, due to the hygroscopic properties of PG and GLY, could lead to dehydration of the airway surface liquid, resulting in decreased mucocilliary clearance, airway obstruction and inflammation.
For e-cigarettes containing nicotine animal experiments have shown increased airway hyperreactivity and an impaired ciliary beat frequency. The main cytokines involved were IL-6 and IL-8 (54).
An indirect effect may be the release of proinflammatory cytokines, with subsequent microvascular leakage (55,56). The observed respiratory insufficiency in EVALI patients might also be explained by this molecular mechanism, in which the above-described effects could interfere with alveolar surfactant, resulting in additional collapse of the small airways (57,58).
Although still speculative and not proven in clinical studies yet, this paradigm of epithelial dysfunction with impaired respiratory fluid regulation (due to capillary leakage and decreased surfactant) might link the described molecular reactions to the radiological findings in patients with EVALI, especially in case of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS).
The cascade starting with the release of proinflammatory cytokines would justify the high doses of corticosteroids in patients with EVALI.
Follow-up after hospitalization
There is no uniform protocol that should be followed after hospital discharge. The CDC recommend a follow-up visit within 1–2 weeks, including pulse-oximetry, and a chest X-ray follow-up examination. An additional follow-up visit should include spirometry, CO-diffusion capacity and chest X-ray after 1–2 months. In the study of Blagev et al. reporting on 26 patients who were followed up within 2 weeks, it was noted that, despite clinical and radiographic improvement in all patients, 67% of patients had residual abnormalities on chest radiographs and 67% had abnormalities in the pulmonary function tests (24). At each follow-up visit patients should be advised to refrain from e-cigarette consumption.
Prognosis and case fatality rate
The prognosis is relatively good, even in severe disease, although fatalities have been described (see above). The mean duration of hospitalization overall was 6.7 days; in the age group of ≥51 years it was 14.8 days (19). Of the patients with readmissions to the hospital within 2 weeks after treatment for EVALI, 50% had relapsed with e-cigarette consumption.
Long-term complications, risk of recurrence and modifying factors such as genetic vulnerability are currently not known. The Lung Injury Response Clinical Working Group observed relapses both during corticosteroid tapering and with resumption of e-cigarettes use (personal communication) (19). After prolonged corticosteroid treatment, some patients might need endocrinological follow-up to monitor adrenal function. The post-treatment/discharge monitoring of patients with EVALI will probably reveal interesting long-term information but has yet to be established.
Conclusions
EVALI is a relatively new syndrome associated with e-cigarette consumption, with a wide variety of pulmonary, gastrointestinal and constitutional symptoms. Increased awareness among physicians is needed to adequately diagnose patients with EVALI and in order to start treatment as soon as possible. Immediate and complete cessation of vaping, high doses of systemic corticosteroids, appropriate antibacterial and antiviral management and identification of patients with bronchiolitis in order to treat them with macrolides, are crucial in an early stage of EVALI. This may pose a challenge due to the widespread use of e-cigarettes and some common symptoms shared with viral pulmonary infections, such as influenza infection. In addition, it is not common practice yet to ask about vaping in routine history taking by clinicians despite the importance of this task in recognizing potential EVALI patients. A multidiscipline approach with pulmonology, radiology, intensive care medicine, microbiology, pathology, toxicology, psychology and sometimes also thoracic surgery is needed in the work-up of suspected EVALI cases.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Breitbarth AK, Morgan J, Jones AL. E-cigarettes: an unintended illicit drug delivery system. Drug Alcohol Depend 2018;192:98-111. [Crossref] [PubMed]
- Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. N Engl J Med 2020;382:903-16. [Crossref] [PubMed]
- Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of Bronchoalveolar Lavage Fluid from Patients in an Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - 10 States, August-October 2019. MMWR Morb Mortal Wkly Rep 2019;68:1040-1. Erratum in: Erratum: Vol. 68, No. 45. [MMWR Morb Mortal Wkly Rep. 2020]. [Crossref] [PubMed]
- Hage R, Fretz V, Schuurmans MM. Electronic cigarettes and vaping associated pulmonary illness (VAPI): a narrative review. Pulmonol 2020. https://doi.org/ [Crossref]
- Sakla NM, Gattu R, Singh G, et al. Vaping-associated acute respiratory distress syndrome. Emerg Radiol 2020;27:103-6. [Crossref] [PubMed]
- Casanova GS, Amaro R, Soler N, et al. An important case of e-cigarette or vaping associated lung injury (EVALI) in Barcelona. Eur Respir J 2020;55:1902076. [Crossref] [PubMed]
- Landman ST, Dhaliwal I, Mackenzie CA, et al. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ 2019;191:E1321-31. [Crossref] [PubMed]
- Flower M, Nandakamur L, Singh M, et al. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system conformed with open lung biopsy. Respirol Case Rep 2017;5:e00230. [Crossref] [PubMed]
- Sommerfeld CG, Weiner DJ, Nowalk A, et al. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics 2018;141:e20163927. [Crossref] [PubMed]
- Moore K, Young II H, Ryan MF. Bilateral pneumonia and pleural effusions subsequent to electronic cigarette use. Open J Emerg Med 2015;3:18-22. [Crossref]
- Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J 2018;12:1295-9. [Crossref] [PubMed]
- Agustin M, Yamamoto M, Cabrera F, et al. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol 2018;2018:9724530. [Crossref] [PubMed]
- Sharma M, Anjum H, Bulathsinghala C, et al. A case report of secondary spontaneous pneumothorax induced by vape. Cureus 2019;11:e6067. [PubMed]
- Buus D, Alzoubaidi M, Jamous F. Vaping induced lung injury: a case report. S D Med 2019;72:446-9. [PubMed]
- Qarajeh R, Kitchen J. THC Vaping-Induced Acute Respiratory Distress Syndrome. Am J Med 2020;133:e147-8. [PubMed]
- Abeles M, Popofsky S, Wen A, et al. Vaping-associated lung injury caused by inhalation of cannabis oil. Pediatr Pulmonol 2020;55:226-8. [Crossref] [PubMed]
- Ocampo-Gonzalez FA, Park JW. Cytologic features of vaping-induced lung injury: A case report. Diagn Cytopathol 2020;48:174-6. [Crossref] [PubMed]
- Singh A, Tan Q, Saccone NM, et al. A Case of Vaping TCH Oil Leading to Vaping Associated Pulmonary Injury: Our Approach to Its Diagnosis, Management, and Recommendations. Case Rep Pulmonol 2020;2020:6138083. [Crossref] [PubMed]
- Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: Interim Guidance for Health Care Providers Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use Associated Lung Injury - United States, October 2019. MMWR Morb Mortal Wkly Rep 2019;68:919-27. [Crossref] [PubMed]
- Atkins G, Drescher F. Acute inhalational lung injury related to the use of electronic nicotine delivery system (ENDS). Chest 2015;148:83A. [Crossref]
- Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med 2019;381:1488-9. [Crossref] [PubMed]
- Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med 2019;7:1017-26. [Crossref] [PubMed]
- Viswam D, Trotter S, Burge PS, et al. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep 2018. [Crossref] [PubMed]
- Blagev DP, Harris D, Dunn AC, et al. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet 2019;394:2073-83. [Crossref] [PubMed]
- Anderson RP, Zechar K. Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep 2019;26:171-3. [Crossref] [PubMed]
- CDC. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Available online: (accessed on November 9th, 2019).https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#key-facts-vit-e
- Chand HS, Muthumulage T, Mazaik W, et al. Pulmonary toxicity and the pathophysiology of electronic cigarette, or vaping product, use associated lung injury. Front Pharmacol 2020;10:1619. [Crossref] [PubMed]
- Blount BC, Karwowski MP, Shields PG, et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med 2020;382:697-705. [Crossref] [PubMed]
- Xue Y, Williams TL, Li T, et al. Type II pneumocytes modulate surfactant production in response to cigarette smoke constituents: restoration by vitamins A and E. Toxicol In Vitro 2005;19:1061-9. [Crossref] [PubMed]
- Beattie JR, Schock BC. Identifying the spatial distribution of vitamin E, pulmonary surfactant and membrane lipids in cells and tissue by confocal Raman microscopy. Methods Mol Biol 2009;579:513-35. [Crossref] [PubMed]
- Thakrar PD, Boyd KP, Swanson CP, et al. E-cigarette, or vaping, product use-associated lung injury in adolescents: a review of imaging features. Pediatr Radiol 2020;50:338-44. [Crossref] [PubMed]
- Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med 2019;381:1486-7. [Crossref] [PubMed]
- Drabkin MJ, Heyligers B. Vaping-associated pulmonary disease (VAPD): an unusual pattern of CT findings. Radiol Case Rep 2019;15:154-5. [Crossref] [PubMed]
- Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J 2013;41:402-9. [Crossref] [PubMed]
- Itoh M, Aoshiba K, Herai Y, et al. Lung injury associated with electronic cigarettes inhalation diagnosed by transbroncial lung biopsy. Respirol Case Rep 2017;6:e00282. [Crossref] [PubMed]
- Choi JW, Lee KS, Chung MP, et al. Giant cell interstitial pneumonia: high-resolution CT and pathologic findings in four adult patients. AJR Am J Roentgenol 2005;184:268-72. [Crossref] [PubMed]
- Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med 2004;350:800-8. [Crossref] [PubMed]
- Henry TS, Kligerman SJ, Raptis CA, et al. Imaging Findings of Vaping-Associated Lung Injury. AJR Am J Roentgenol 2020;214:498-505. [Crossref] [PubMed]
- Hess IM, Lachireddy K, Capon A. A systematic review of the health risks from passive exposure to electronic cigarette vapour. Public Health Res Pract 2016. [Crossref] [PubMed]
- Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med 2014;47:15-7. [Crossref] [PubMed]
- Dicpinigaitis PV, Trachuk P, Fakier F, et al. Vaping-Associated Acute Respiratory Failure Due to Acute Lipoid Pneumonia. Lung 2020;198:31-3. [Crossref] [PubMed]
- Modi S, Sangani R, Alhajhusain A. Acute lipoid pneumonia secondary to e-cigarette use: an unlikely replacement for cigarettes. Chest 2015;148:382A. [Crossref]
- Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med 2019;381:1780-1. [Crossref] [PubMed]
- Lansbury L, Rodrigo C, Leonardi-Bee J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2019;2:CD010406. [PubMed]
- Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 2016;94:667-79. [Crossref] [PubMed]
- Miyashita L, Suri R, Dearing E, et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J 2018;51:1701592. [Crossref] [PubMed]
- Qasim H, Karim Z, Silva-Espinoza J, et al. Short-term e-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. J Am Heart Assoc 2018;7:e009264. [Crossref] [PubMed]
- Evans ME, Twentyman E, Click ES, et al. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use-Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge - United States, December 2019. MMWR Morb Mortal Wkly Rep 2020;68:1189-94. [Crossref] [PubMed]
- Gilpin DF, McGown KA, Gallagher K, et al. Electronic cigarette vapour increases virulence and inflammatory potential of respiratory pathogens. Respir Res 2019;20:267. [Crossref] [PubMed]
- Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 2015;10:e0116732. [Crossref] [PubMed]
- Schweitzer KS, Chen SX, Law S, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 2015;309:L175-87. [Crossref] [PubMed]
- Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015;10:e0116861. [Crossref] [PubMed]
- Chaumont M, van de Borne P, Bernard A, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol 2019;316:L705-19. [Crossref] [PubMed]
- Garcia-Arcos I, Geraghty P, Baumlin N, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016;71:1119-29. [Crossref] [PubMed]
- Fowles JR, Banton MI, Pottenger LH. A toxicological review of the propylene glycols. Crit Rev Toxicol 2013;43:363-90. [Crossref] [PubMed]
- Iskandar AR, Gonzalez-Suarez I, Majeed S, et al. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol Mech Methods 2016;26:389-413. [Crossref] [PubMed]
- Przybyla RJ, Wright J, Parthiban R, et al. Electronic cigarette vapor alters the lateral structure but not tensiometric properties of calf lung surfactant. Respir Res 2017;18:193. [Crossref] [PubMed]
- Sosnowski TR, Jablczynska K, Odziomek M, et al. Physicochemical studies of direct interactions between lung surfactant and components of electronic cibgarettes liquid mixtures. Inhal Toxicol 2018;30:159-68. [Crossref] [PubMed]


