Combination of apatinib and docetaxel in treating advanced non-squamous non-small cell lung cancer patients with wild-type EGFR: a multi-center, phase II trial
Introduction
Lung cancer is the most prevalent and fatal cancer in Chinses population with the non-small cell lung cancer (NSCLC), accounting for 80–85% of all lung cancer cases and being the most significant subtype (1). NSCLC is further divided into squamous cell carcinoma, large cell carcinoma, adenocarcinoma and others, among which non-squamous NSCLC accounts for half of all NSCLC cases. In clinical settings, most NSCLC patients are diagnosed as locally advanced or systematically metastasized diseases; thus, curative surgery is unprocurable. Instead, the targeted therapy for patients with targetable genetic aberrations [such as epidermal growth factor receptor (EGFR)] improves the survival of advanced NSCLC patients. However, for patients lacking targetable genetic aberrations, chemotherapy remains the only option (2,3). Although chemotherapy, as first-line option, has brought certain benefits and there exists the choice of second-line therapy, the clinical outcomes for advanced NSCLC patients are barely satisfactory, especially when the patients fail the first-line therapy.
In various malignancies, angiogenesis is an essential mechanism for tumor growth, metastasis and recurrence. Among cancer therapeutic strategies, anti-angiogenesis agents, in combination with chemotherapy or not, have shown survival benefits in advanced-stage malignancies (4). Vascular epidermal growth factor receptor 2 (VEGFR2) is specifically responsible for angiogenesis functioning via regulating multiple signaling pathways (5). Therefore, VEGFR2 inhibitors including receptor-specific antibodies and small molecule drugs have been developed, and a few of them have been approved as standard treatment for advanced malignancies (4). Apatinib is a small molecular vascular epidermal growth factor (VEGF) tyrosine kinase inhibitor (TKI) that binds and targets the intracellular domain of VEGFR2. It has been used in advanced malignancy patients who fail the prior chemotherapy and other targeted therapies (6). The use of apatinib has been approved by FDA and cFDA for advanced gastric cancer, and supportive data from several randomized, double-blinded, placebo-controlled clinical trials illustrate that apatinib significantly improves progression-free survival (PFS) and overall survival (OS) with acceptable safety in patients with advanced gastric cancer including those who have failed two or more lines of chemotherapy (7,8).
As for advanced NSCLC, favorable survival outcomes are observed after treated with apatinib, and the drug-related toxicity is tolerable and controllable (9-11). Also, the combination of apatinib with cytotoxic drugs such as docetaxel has been proven effective in treating advanced NSCLC (12). In a previous multi center prospective study, apatinib plus docetaxel achieves ORR of 33.33%, DCR of 66.67% and median PFS of 2.92 months as the second or above line treatment in advanced non-squamous NSCLC (12). However, whether apatinib combining docetaxel would improve the treatment outcomes of advanced non-squamous NSCLC patients with wild-type EGFR is seldom reported, only a phase I trial shows promising safety and treatment response to apatinib plus docetaxel in 12 advanced lung adenocarcinoma patients with wild-type EGFR (13). Therefore, in this multi-center, phase II trial, we aimed to investigate the treatment response, survival profiles and treatment-related adverse events (AEs) of apatinib plus docetaxel in treating advanced non-squamous NSCLC patients with wild-type EGFR.
Methods
Patients
This single-arm, multi-center, phase II trial prospectively recruited 30 advanced non-squamous NSCLC patients with wild-type EGFR, and all eligible patients met following inclusion criteria: (I) histologically diagnosed as advanced non-squamous NSCLC (stage IIIB or IV) with wild-type EGFR confirmed by EGFR mutation testing; (II) age ≥18 years; (III) had at least one measurable lesion defined by Response Evaluation Criteria in Solid Tumors (RECIST); (IV) disease progression after treatment with platinum-based chemotherapy regimen; (V) Eastern Cooperative Oncology Group performance status (ECOG PS) score 0 or 1; (VI) a life expectancy ≥3 months; (VII) adequate hematologic, hepatic, and renal function; (VIII) willingness to practice contraception during the trial. The key exclusion criteria included: (I) squamous carcinoma (including adenosquamous carcinoma) or small cell lung carcinoma; (II) history or newly diagnosed central nervous system metastases; (III) intratumor cavitation or necrosis; (IV) major blood vessel involvement; (V) uncontrolled hypertension; (VI) unstable angina, myocardial infarction, and class III or IV congestive heart failure as defined by the New York Heart Association (NYHA); (VII) suffered from thromboembolic events within the latest 12 months, including cerebrovascular accident, deep venous thrombosis and pulmonary thrombosis; (VIII) history of hemoptysis (more than one-half teaspoon of bright red blood per day, within the preceding 2 months); (IX) bleeding tendency or current treatment with coagulation therapy; (X) incapable of oral intake; (XI) intestinal paralysis or ileus; (XII) confirmed pregnancy or lactation period.
Ethics statement
This phase II trial (Chinese Clinical Trial Registry Number: ChiCTR1800020105) was approved by the Ethics Committee of our hospital (Ethical Approval Number: 2016NZKY-014-01) and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonization. All patients signed the informed consents prior to enrollment and were voluntary to comply with treatment protocol, follow-up assessments and procedures.
Baseline data collection
After enrollment, patients’ baseline characteristics were documented, which included age, sex, ECOG PS score, number of metastatic sites, prior treatments (surgery, chemotherapy, radiotherapy, target therapy, first-line treatment regimen), prior lines of treatments, and PFS from first-line treatment.
Treatment
All patients received combined therapy of apatinib (Jiangsu Hengrui Medicine Co., Ltd, China) and docetaxel (Jiangsu Hengrui Medicine Co., Ltd, China) (Table S1). The apatinib was administered as follows: oral dose of 500 mg once daily until intolerable toxicity, disease progression, or death. A continuous 28-day treatment was defined as one treatment cycle. Dose reduction to 250 mg once daily of apatinib was allowed if patients experienced grade 3/4 hematologic AEs, or other non-hematologic AEs that the investigators considered it was necessary to reduce dose. The docetaxel was administered at dose of 60 mg/m2 intravenously over 1 h, repeated every 3 weeks which was defined as one treatment cycle, and continued for 4–6 cycles. Treatment cessation criteria for this trial were: (I) subjects withdrew their informed consents; (II) disease progression confirmed by imaging examination; (III) subjects were still intolerable to the toxicity after dose adjustment; (IV) other circumstances where the researcher considered it necessary for subjects to withdraw from the trial. Besides, after trial discontinuation, apatinib plus docetaxel or combined chemotherapy was allowed for patients with disease progression at the discretion of the investigators and patients.

Full table
Endpoints and outcome evaluation
The primary endpoint was PFS. Secondary endpoints included OS, objective response rate (ORR) and disease control rate (DCR) at first evaluation after one-month treatment, and AEs. Since starting therapy, all patients were followed-up monthly by phone call or clinic consultation according to the scheduled protocol, and the survival status and AEs were documented, which was lasted until death, patient dropping-out or the end of the trial. The PFS was calculated from the date of initial treatment by apatinib and docetaxel to the date of disease progression or death (whichever occurred first). OS was calculated from the date of initial treatment by apatinib and docetaxel to the date of death. Treatment response was evaluated according to RECIST 1.1, which was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was defined as CR + PR, and the DCR was defined as CR + PR + SD. First tumor response assessment was performed at one-month treatment of apatinib and docetaxel by the institutions’ radiology group using enhanced computed tomographic or magnetic resonance imaging scan. AEs were assessed and graded following the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.0) and classified as degree 0–4.
Statistical analysis
Twenty-nine patients were included in response and survival analysis, and one patient without response and survival assessments due to early lost follow-up was excluded. While all 30 patients were included in the safety analysis. Descriptive analysis was displayed as mean and standard (SD), median and interquartile range (IQR), or number and percentage [No. (%)]. PFS and OS were illustrated by Kaplan-Meier survival curves. Statistical analysis was performed using SPSS 22.0 software (IBM, USA).
Results
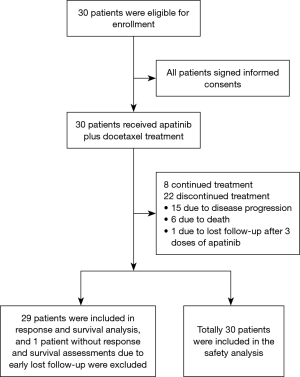
Study flow
Thirty NSCLC patients were eligible for enrollment, and signed the informed consents (Figure 1). All patients received apatinib plus docetaxel treatment, and 8 of them continued treatment but 22 of them discontinued due to disease progression (n=15), death (n=6), lost follow-up after 3 doses of apatinib (n=1). Finally, 29 patients were included in response and survival analysis, while 1 patient without response and survival assessment due to early lost follow-up was excluded; meanwhile, all 30 patients were included in the safety analysis.
Baseline characteristics
The enrolled NSCLC patients were aged 60.17±9.79 years in average with 19 (63.3%) being males and 11 (36.7%) being females (Table 1). There were 5 (16.7%) patients with ECOG PS score 0 and 25 (83.3%) patients with ECOG PS score 1. Number of patients with 0, 1, 2 and 3 metastatic sites was 8 (24.7%), 5 (16.7%), 13 (43.3%) and 4 (13.3%) respectively. The mean/median PFS from first-line treatment were 5.93±5.62/4.43 (1.97–6.40) months. Other clinical characteristics of NSCLC patients were shown in Table 1.

Full table
Treatment response
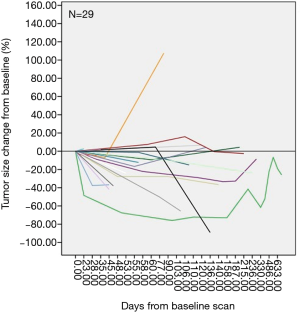
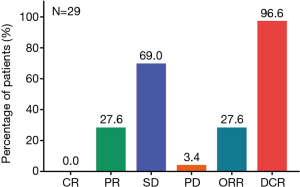
Since one patient lacked response and survival assessment due to early lost follow-up, 29 patients were included in treatment response and survival analysis. Assessment of treatment response revealed that no patient achieved CR (0.0%), 8 patients achieved PR (27.6%), 20 patients were with SD (69.0%), and 1 patient were with PD (3.4%), resulting in ORR and DCR of 27.6% and 96.6%, respectively (Figure 2). Besides, tumor size was recorded at each examination and the change of tumor size compared with baseline of each patient was shown in Figure 3.
Survival profile
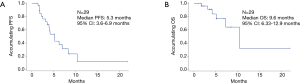
All patients were followed-up monthly by phone call or clinic consultation, and the survival status were documented. As calculated, the median PFS was 5.3 months (95% CI: 3.6–6.9 months) (Figure 4A), and the median OS was 9.6 months (95% CI: 6.33–12.9 months) (Figure 4B).
Treatment-related AEs
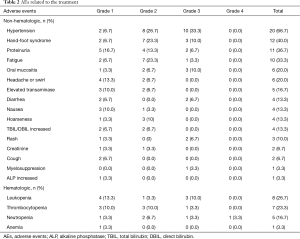
The common (defined as incidence >10.0%) non-hematologic AEs included: hypertension (66.7%), hand-foot syndrome (40.0%), proteinuria (36.7%), fatigue (33.3%), oral mucositis (20.0%), headache or swirl (20.0%), elevated transaminase (16.7%), diarrhea (13.3%), nausea (13.3%), hoarseness (13.3%) and TBIL/DBIL increased (13.3%) (Table 2). The common (defined as incidence >10.0%) hematologic AEs were leukopenia (26.7%), thrombocytopenia (23.3%) and neutropenia (16.7%) (Table 2). Notably, most of the AEs were at grade 1–2, and a minor proportion were at grade 3, while only one case was at grade 4 (neutropenia). Meanwhile, most of the AEs were tolerable.
Full table
Discussion
Apatinib has been approved in China as the third-line treatment for advanced gastric cancer, and it is under clinical trials for other advanced malignancies including advanced NSCLC. For advanced NSCLC patients who fail the first or above chemotherapy/targeted therapy, the use of apatinib shows promising efficacy and manageable toxicity (14,15). In a pooled analysis of 14 studies including a total of 457 advanced NSCLC patients, the pooled median PFS is 4.77 months and the pooled median OS is 6.85 months; in addition, the pooled ORR and DCR values are 18% and 72% respectively (14). Another study reports that apatinib achieves ORR and DCR of 6.9% and 67.4%, respectively; and a median PFS as well as median OS of 3.8 months and 5.8 months respectively in 52 advanced NSCLC patients who progress after second-line or more treatments (16). In addition, in 100 stage III/IV NSCLC patients, the ORR and DCR to apatinib as third or subsequent-line therapy are 11.0% and 67.0% respectively; the median PFS is 2.93 months (15).
Docetaxel is recommended as the second-line chemotherapy for advanced NSCLC patients with wild-type EGFR (17). Previous report reveals the median PFS and OS to docetaxel as 4.2 and 9.4 months respectively in advanced non-squamous NSCLC patients (18). Despite that, the combination of anti-angiogenesis receptor TKI with docetaxel improves PFS in advanced NSCLC patients. For instance, in a phase III, double-blind and randomized-controlled clinical trial, nintedanib (anti-angiogenesis receptor TKI) in combination with docetaxel notably improves PFS and OS compared with docetaxel plus placebo in patients with previously treated NSCLC, especially in those with adenocarcinoma (19). Additionally, combination of apatinib with chemotherapy agents, yields better treatment outcomes, including inhibiting the proliferation of NSCLC cells, reducing the micro vessel density and anti MAPK-ERK and PI3K-AKT-mTOR signaling pathway activation compared with apatinib monotherapy in NSCLC mouse xenograft model (20). These studies illustrate that there may be a synergizing effect of anti-angiogenesis receptor TKI combined with cytotoxic drugs in treating advanced NSCLC.
As for the combination of apatinib and docetaxel, the median PFS duration for this patient is 4.2 months in heavily pretreated advanced non-squamous NSCLC patients treated with apatinib plus docetaxel, which is favorable numerically referring to the previous studies (11). Furthermore, in a previous multi center prospective study, apatinib plus docetaxel achieves ORR of 33.33%, DCR of 66.67% and median PFS of 2.92 months as the second or above line treatment in advanced non-squamous NSCLC (12). Whereas for advanced non-squamous NSCLC patients with wild-type EGFR, the treatment efficacy of apatinib plus docetaxel is uninvestigated until a phase I trial shows safety and tolerability of apatinib plus docetaxel in treating a small number of advanced lung adenocarcinoma patients with wild-type EGFR. Thus, we investigated the efficacy and safety of apatinib plus docetaxel in treating advanced non-squamous NSCLC patients with wild-type EGFR in this multi-center, phase II trial. This study observed that the ORR and DCR were 27.6% and 96.6% respectively; the median PFS was 5.3 months, and the median OS was 9.6 months in advanced non-squamous NSCLC patients with wild-type EGFR treated with apatinib plus docetaxel. Numerically, the median PFS and OS were higher compared with that of apatinib or docetaxel alone in previous studies, as well as the superior rate of ORR and DCR. This might due to the synergizing effect of apatinib and docetaxel, while the detailed mechanism was not investigated in this study.
The common apatinib-related AEs include hypertension, proteinuria, hand-foot-skin reaction, fatigue, oral mucositis and hematologic toxicity. In a clinical trial using apatinib as the second-line treatment for advanced NSCLC, the incidences of AEs are hypertension (53%), hand-foot reaction (47%), proteinuria (41%), fatigue (21%), anorexia (19%), and hematologic toxicity (15%); and no grade 4 AE is observed (15). For the AEs with higher grade (grade 3/4), a previous pooled analysis reports grade 3/4 hypertension (7%), proteinuria (3%), hand-foot-skin reaction (6%), fatigue (4%), decreased appetite (1.1%), oral mucositis (3%), and thrombocytopenia (3%) in advanced NSCLC patients treated with apatinib alone (14). Regarding docetaxel, the related AEs are mostly neutropenia, fatigue, nausea and alopecia. Since this study used apatinib plus docetaxel, the common treatment-related AEs included hypertension (66.7%), hand-foot syndrome (40.0%), proteinuria (36.7%), fatigue (33.3%), oral mucositis (20.0%), headache or swirl (20.0%); and hematologic toxicities including leukopenia (26.7%), thrombocytopenia (23.3%) and neutropenia (16.7%). Most AEs were at grade 1–2, and there existed only 1 case of grade 4 AE which was neutropenia. Taken together, the common AEs of apatinib plus docetaxel were predictable according to the AEs of apatinib or docetaxel alone, and that the high-grade AEs were rare, indicating that apatinib plus docetaxel had reasonable and controllable safety profiles in treating advanced non-squamous NSCLC with wild-type EGFR.
As a single-arm, multi-center, phase II trial, this study existed several shortcomings. The sample size was relatively small (N=30), which might slightly reduce statistical power. In addition, since this was a single-arm trial, whether apatinib plus docetaxel was comparable to apatinib/docetaxel alone required further validation by randomized, controlled trial; and whether there existed synergizing effect of apatinib and docetaxel in treating advanced non-squamous NSCLC needed to be investigated by further studies.
In conclusion, apatinib plus docetaxel presents favorable treatment efficacy and tolerable safety in advanced non-squamous NSCLC patients with wild-type EGFR.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (grant number 81570078); National Natural Science Foundation of China (grant number 81770082); National Natural Science Foundation of China (grant number 81772500) and Natural Science Foundation of Jiangsu Province (grant number BK20161386).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.54). YS serves as an unpaid editorial board member of Journal of Thoracic Disease from Mar 2012 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This phase II trial (Chinese Clinical Trial Registry Number: ChiCTR1800020105) was approved by the Ethics Committee of our hospital (Ethical Approval Number: 2016NZKY-014-01) and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonization. All patients signed the informed consents prior to enrollment and were voluntary to comply with treatment protocol, follow-up assessments and procedures.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu D, Liang L, Nie L, et al. Efficacy, safety and predictive indicators of apatinib after multilines treatment in advanced nonsquamous nonsmall cell lung cancer: Apatinib treatment in nonsquamous NSCLC. Asia Pac J Clin Oncol 2018;14:446-52. [Crossref] [PubMed]
- Minami S, Kijima T. Pemetrexed in maintenance treatment of advanced non-squamous non-small-cell lung cancer. Lung Cancer (Auckl) 2015;6:13-25. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91. [Crossref] [PubMed]
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc) 2015;51:223-9. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Liu Z, Ou W, Li N, et al. Apatinib monotherapy for advanced non-small cell lung cancer after the failure of chemotherapy or other targeted therapy. Thorac Cancer 2018;9:1285-90. [Crossref] [PubMed]
- Ding L, Li QJ, You KY, et al. The Use of Apatinib in Treating Nonsmall-Cell Lung Cancer: Case Report and Review of Literature. Medicine (Baltimore) 2016;95:e3598. [Crossref] [PubMed]
- Song Z, Yu X, Lou G, et al. Salvage treatment with apatinib for advanced non-small-cell lung cancer. Onco Targets Ther 2017;10:1821-5. [Crossref] [PubMed]
- Jiang Q, Zhang NL, Ma DY, et al. Efficacy and safety of apatinib plus docetaxel as the second or above line treatment in advanced nonsquamous NSCLC: A multi center prospective study. Medicine (Baltimore) 2019;98:e16065. [Crossref] [PubMed]
- Duan JC, Wang ZJ, Lin L, et al. Apatinib, a novel VEGFR inhibitor plus docetaxel in advanced lung adenocarcinoma patients with wild-type EGFR: a phase I trial. Invest New Drugs 2019;37:731-7. [Crossref] [PubMed]
- Ma JT, Sun J, Sun L, et al. Efficacy and safety of apatinib in patients with advanced nonsmall cell lung cancer that failed prior chemotherapy or EGFR-TKIs: A pooled analysis. Medicine (Baltimore) 2018;97:e12083. [Crossref] [PubMed]
- Zhang D, Zhang C, Huang J, et al. Clinical investigation of the efficacy and toxicity of apatinib (YN968D1) in stage III/IV non-small cell lung cancer after second-line chemotherapy treatment: A retrospective study. Thorac Cancer 2018;9:1754-62. [Crossref] [PubMed]
- Leng J, Li DR, Huang LM, et al. Apatinib is effective as third-line and more treatment of advanced metastatic non-small-cell lung cancer: A retrospective analysis in a real-world setting. Medicine (Baltimore) 2019;98:e16967. [Crossref] [PubMed]
- Xu J, Ding G, Zhang X, et al. The EGFR tyrosine kinase inhibitors as second-line therapy for EGFR wild-type non-small-cell lung cancer: a real-world study in People's Republic of China. Onco Targets Ther 2016;9:6479-84. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Liu M, Wang X, Li H, et al. The effect of apatinib combined with chemotherapy or targeted therapy on non-small cell lung cancer in vitro and vivo. Thorac Cancer 2019;10:1868-78. [Crossref] [PubMed]