
Direct and indirect CT imaging features of esophago-airway fistula in adults
Introduction
Esophago-airway fistulas (EAF) include both tracheoesophageal (TEF) and bronchoesophageal fistulas (BEF) and are pathological connections between the esophagus and the trachea or a major bronchus. While EAF are rare, they cause significant morbidity and mortality due to recurrent respiratory infections and malnutrition (1,2).
When diagnosed in adulthood, the EAF is typically acquired. Malignancies, most commonly esophageal cancer followed by lung cancer, account for more than half of the cases in the adult (3,4). Benign conditions such as prolonged intubation, trauma (both iatrogenic and non-iatrogenic), and infections are other less common causes of acquired EAF (1,5-9). Even less frequent are small congenital TEFs that are diagnosed or recur late in adulthood (10-12).
The contrast esophagogram, typically using barium, remains the primary radiographic tool for the diagnosis of TEF and BEF. Computed tomography (CT) is also often employed in cases of suspected or proven fistulas in adults to further characterize the fistula and the esophageal and airway anatomy, to evaluate for complications including mediastinal and lung infections, and as an alternative in patients who are unable to swallow or unable to cooperate or tolerate fluoroscopy, such as those with altered mentation or on ventilator support. Sometimes, previously undiagnosed and unsuspected EAF can be detected on CT performed in patients presenting with respiratory symptoms underscoring the importance for radiologists to be familiar with CT findings of this entity (13,14).
While the findings of TEF and BEF on contrast esophagography are well-described, to our knowledge, a systematic analysis of CT findings of EAF in adults has not been previously published. The goal of our study was to determine the direct and indirect CT findings of EAF in adults.
Methods
Patient identification and inclusion
The institutional review board approved this study, with informed consent waived due to its retrospective nature (IRB# 2018P003136). We queried our institutional radiology information system for chest CT examinations performed between January 2001 and December 2019 at a major academic center with reports containing “tracheoesophageal fistula” or “bronchoesophageal fistula” in the indication, body, or impression of the report. The patients with confirmed EAF on endoscopy, or surgery were selected for review. The CT closest to the date of confirmatory study and before the intervention was selected for review, if multiple scans were performed.
Review of CT imaging studies
CT examinations were performed on multidetector row CT scanners from different vendors, with a slice thickness of 1.25 to 2.5 mm. Unless contraindicated, intravenous contrast was routinely administered and, in 6/26 cases, oral contrast was also administered. Three radiologists with subspecialty training in thoracic imaging (SRD, BPL, and DPM) reviewed the studies independently using the institutional picture archiving and communications system (AGFA Impax 6, Mortsel, Belgium). Disagreements, if any, were resolved by consensus.
The EAF findings on CT were categorized as direct or indirect. Identification of a tract between the esophagus and trachea or bronchus constituted a direct sign. The size (width and length) and location of the fistulous connection and tract contents (air, fluid, or both) were recorded.
The indirect findings were assessed in the airway, esophagus, mediastinum, and lungs. The airway findings included tracheal and bronchial wall thickening >4 mm (15), dilation of the trachea, and debris or fluid within the central airways. The esophageal findings included wall thickening >4 mm (16), esophageal dilatation with air, extravasation of oral contrast into either airways and/or mediastinum (when oral contrast was given), presence of a sinus tract arising from the esophagus but not appreciably connecting to the trachea, and gastric distention. The mediastinal findings were fat stranding, extraluminal air or fluid, and lymphadenopathy (lymph nodes >1 cm in the short axis). The indirect lung findings were consolidations, ground glass opacities, and bronchocentric nodules, suggestive of aspiration or other pneumonia.
Statistical analysis
Patient characteristics and imaging features of the fistulas were analyzed using descriptive statistics. The features of benign and malignant causes of EAF were compared using the Wilcoxon rank-sum test and Fisher’s exact test for continuous and categorical variables, respectively. All tests were two-sided. A P value of 0.05 or less was considered significant.
Results
Patient characteristics
We identified 26 patients with CT studies with TEF or BEF and with endoscopic or surgical confirmation (Table 1). Seven patients also had a contrast esophagogram performed prior to the CT as part of the workup; of these, EAF was identified in 4/7 (57.1%), while in 3/7 (42.9%), the fistula could not be demonstrated.
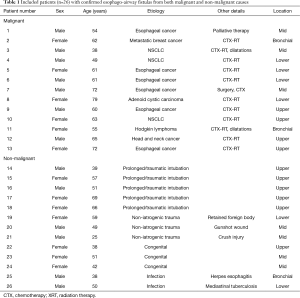
Full table
The clinicopathologic characteristics are summarized on Table 2. Half (13/26; 50%) of the patients had an underlying malignancy. The most common malignancies were esophageal cancer (6/13; 46.2%) and lung cancer (3/13; 23.1%). There were also one each case of head and neck cancer, tracheal adenoid cystic carcinoma, lymphoma, and breast cancer with lung metastases. Most of these patients (11/13; 84.6%) underwent combined chemotherapy and radiation therapy. Most EAF in these patients (9/13; 69.2%) developed after cancer-related treatments, while the remainder (4/13; 30.8%) were found before treatment due to direct tumor extension.
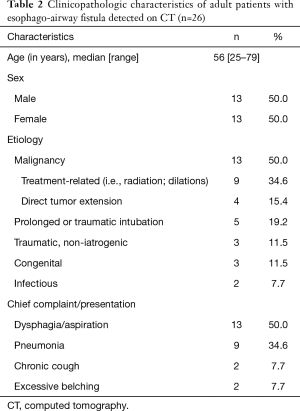
Full table
The second most common etiology for EAF formation was prolonged or traumatic intubation (5/26; 19.2%). Other etiologies included non-iatrogenic trauma (3/26; 11.5%), congenital-developmental anomaly (3/26; 11.5%), and infection (2/26; 7.7%). Non-iatrogenic traumatic etiologies included a transmediastinal gunshot wound, a foreign body retained in the esophagus, and a reported remote “crush” injury. Infectious causes included mediastinal tuberculosis and herpes esophagitis in the setting of HIV infection.
Most patients (17/26) were diagnosed in an inpatient setting. In those that were diagnosed as in the outpatient setting (9/26), three were congenital, three were due to non-iatrogenic trauma, two were due to prolonged intubation, and one was due to infection. Although there was a significant overlap in the symptoms and presentation of patients, the most common presenting symptoms in half of the patients were dysphagia and aspiration (13/26; 50%). While most (22/26, 84.6%) of the TEF were known or suspected at the time of CT, 4 (15.4%) were detected and diagnosed by CT prior to any clinical suspicion.
CT imaging features of fistulas
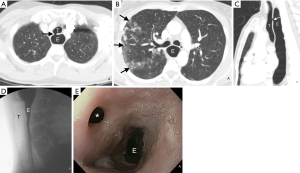
Direct and indirect imaging features identified on CT are presented in Table 3. The fistula was most commonly between the trachea and esophagus (23/26; 88.5%) and most commonly arose from the upper trachea (10/26; 38.5%), followed by the mid- (7/26; 26.9%) and lower trachea (6/26; 23.1%). The BEFs were less frequent (3/26; 11.5%). A direct tract between the esophagus and adjacent airway was identified in most cases (22/26; 84.6%). In the three patients in whom the barium esophagography was negative, a direct connection between the airway and esophagus was could be identified (Figure 1). When identified, the tract mostly contained air (12/22; 54.5%) or both air and fluid (8/22; 36.4%), rather than fluid alone (7/22; 31.8%). When oral contrast was administered (6/26; 23.1%), extra-esophageal contrast was noted only in one (16.7%) patient.
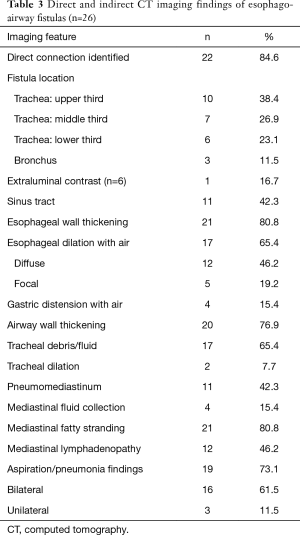
Full table
Inflammatory changes to the esophagus, airway, and mediastinum were common, with esophageal wall thickening and mediastinal fat stranding in 80.8% (21/26) of patients and airway wall thickening in 76.9% (20/26) of patients. Fluid or debris within the airways (17/26; 65.4%) and focal or diffuse esophageal dilation with air (17/26; 65.4%) were also commonly found. Most patients (19/26; 73%) had lung parenchymal abnormalities related to aspiration or other pneumonia. When present, there were typically bilateral (16/19; 84.2%). Pneumomediastinum (11/26; 42.3%) and discrete mediastinal fluid collections (4/26; 15.4%) were, however, less frequently present.
Malignancy-related versus benign fistulas
A comparison of patients with malignancy-related and non-malignancy related EAF is presented in Table 4. Those with malignancy-related fistulas tended to be older than those with non-malignant causes (median age, range, 61 years, 38–79 years versus 50 years, 25–69 years; P=0.019). In general, however, there were no statistically significant differences between the two subgroups regarding the imaging features related to the EAF.
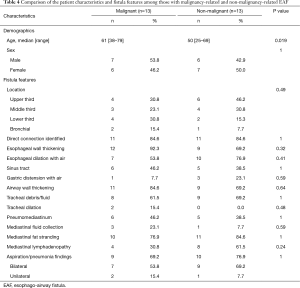
Full table
Discussion
TEF and BEF, although rare in adults, can result in significant morbidity and mortality if left undiagnosed and untreated. CT plays an important role in their diagnosis and characterization. In this study, we determined the direct and indirect findings of TEF and BEF on CT. We found that, in most cases, a direct connection between the esophagus and the airway is seen on CT and that several other ancillary findings may assist in the detection of EAF. Our findings also suggest that CT may be helpful in cases of EAF that are not detected by contrast esophagography and may be the first imaging study to suggest their presence.
Our series consisted of 26 adult patients with EAF found by CT in both benign and malignant settings. Consistent with prior reports, half of our patients had an underlying malignancy (3,4). Notably, EAF developed after or as a sequela of treatment of the malignancy. In those with non-malignant causes, prolonged intubation was the most common cause, as is consistent with prior reports (1,5,6). In 4 cases, CT was the first diagnostic study to suggest or detect the TEF and preceded clinical suspicion and diagnosis.
The CT findings of adult EAF have been previously described only in case reports (11-14,17-21). A small series of 10 congenital fistulas in neonates with esophageal atresia showed a good correlation between CT and surgical findings (22). Several authors have also analyzed the potential role of CT in the management of EAF in children (23-25). However, to our knowledge, a systematic analysis of CT findings of adult EAF has not previously been published.
In our cohort, CT demonstrated a direct connection between the airway and esophagus in the majority (85%) of the patients. When CT and esophagography do not identify a fistulous tract, several indirect imaging features on CT may raise suspicion of an EAF. All 4 patients in whom a fistulous tract was not seen on CT had other indirect signs including esophageal or airway wall thickening or mediastinal fatty stranding (Figures 2 and 3). Other indirect signs seen in most of the patients included esophageal distention with air (Figure 1), fluid or debris within the airways, and lung parenchymal abnormalities suggestive of aspiration or infectious pneumonia (Figure 1). Endoscopy to directly examine the esophagus or tracheobronchial tree is ultimately used to confirm fistulous communication when imaging findings are equivocal in patients with suspected EAF.
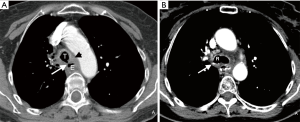
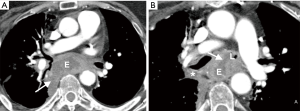
Somewhat surprisingly, oral contrast opacification of the fistulous tract or extra-esophageal extravasation of oral contrast was present in only one of the six cases in which oral contrast was administered (Figure 2). When demonstrated, the extra-esophageal extension of the oral contrast into the airway is diagnostic of EAF; its absence, however, does not exclude a fistula. Failure to demonstrate oral contrast within a fistulous tract at CT might be due to characteristics of the fistula, such as small size or obstructing debris within the tract, transient opening and closing of the fistula, or an acute angle of origin of the fistula preventing the exit of contrast. The method of administration of oral contrast may also play a role in opacification of the tract; administration in the upright position, as was the practice in our series, is likely not as effective as oral contrast administration to a patient in prone or lateral decubitus position due to the more favorable dependent position of the fistula in the latter positions. In addition, given that most fistulas identified on CT contained air or both air and fluid, air may prove to be a helpful contrast agent in detecting these fistulas. As clinically allowable and technically feasible, gentle insufflation of the esophagus or hyperventilation in intubated patients may increase air within the aerodigestive tract and allow for better detection of these fistulas.
Contrast extension from the esophagus to the adjacent airway on contrast esophagogram is diagnostic of EAF (26). Contrast esophagography, however, is not 100% sensitive. The sensitivity and specificity of esophagography in the detection of EAF have yet to be reported, but esophagography has been found to be helpful in detecting leaks following surgery to the neck, esophagus, or stomach, and it is the study of choice in patients presenting with dysphagia following these surgeries (27). Esophagography has been reported to be 36–79% sensitive and 73–97% specific in diagnosing a postoperative leak (28,29). It has also been reported that CT in addition to esophagography can raise sensitivity in detecting postoperative leaks to up to 100% (29). In our cohort, the fistula was not demonstrated in 3 of the 7 patients who underwent contrast esophagography. This observation, however, does not prove that CT is more sensitive than contrast esophagography, as our cohort was selected from a group of patients who had CT for EAF, but it does support the hypothesis that CT can diagnose EAF that are undetectable by esophagography.
Additionally, CT better demonstrates surrounding structures than esophagography, facilitating identification of complications. Mediastinal fluid collections were infrequent and were seen in only 4 of the 26 patients. When present, they may be complicated by an infection. Most patients also had lung parenchymal abnormalities consistent with aspiration and infectious pneumonia that tended to be multifocal when present.
We also sought to compare the imaging features of EAF related to malignancy to those of EAF from non-malignant causes. While it did not reach statistical significance, it is notable that EAF related to malignancy were nearly equally distributed throughout the upper, mid, and lower trachea, while TEFs due to benign causes were slightly more frequently seen in the upper trachea. This variance in distribution was likely driven by TEF secondary to prolonged intubation, all five of which occurred in the upper trachea. TEF from prolonged intubation is thought to result from sustained pressure of the hyper-inflated endotracheal tube cuff against the posterior wall of the trachea, which is pushed against the rigid nasogastric tube in the esophagus, resulting in ischemia, necrosis, and tissue breakdown (30). Several risk factors, including steroid treatment, chronic hypoxia, poor nutrition, sepsis, and prolonged episodes of hypotension, have been associated with TEF formation in chronically intubated patients (30,31).
There were no other significant differences in the imaging features of EAF related to malignant and non-malignant causes. Both groups had high frequencies of identifiable fistulas and esophageal, airway, and mediastinal inflammatory changes. Lymphadenopathy was seen in both groups, with slightly increased (although not statistically significant) frequency in benign EAF, suggesting that these lymph nodes are most likely reactive. Both groups also had a similar incidence of associated lung parenchymal findings of aspiration and other pneumonias.
Definitive surgical repair of acquired EAF should be considered, especially when of benign etiology and with an otherwise reasonable prognosis. CT imaging findings may guide the approach and surgical technique used for repair (32). The level of the fistula, which is well characterized by CT, may dictate whether a cervical or trans-thoracic operation is necessary. Additionally, patients must be optimized before surgical intervention; CT evaluation of the lung parenchyma is helpful to ensure that the pneumonia has been adequately treated.
Our study has limitations. Our retrospective study included patients from a review of CT reports for the presence of an EAF, which could yield selection bias. All of the patients are from a single institution and may limit the generalization of our findings, as there could be variations in scan technique and protocol. Finally, our cohort is small, on account of the rarity of EAF in adults and the variable use of CT for their detection and characterization. Further investigation will be required to evaluate the sensitivity and specificity of findings observed in our study for clinical practice. Nevertheless, our findings show that CT can play an essential role in the evaluation of EAF.
Conclusions
CT is a useful tool in both primary and secondary evaluation of adult EAFs resulting from both malignant and benign etiologies. Although endoscopy is the gold standard for the detection and characterization of TEF and BEF, CT provides accurate characterization of adult EAFs in a wide variety of clinical scenarios, with several characteristic direct and indirect findings. CT may be the first diagnostic exam to suggest and detect the presence of EAFs, preceding clinical suspicion. CT can also detect a subset of fistulas not demonstrated on esophagography and is complimentary to the esophagogram in other cases, providing valuable information about the extent of the fistula and related complications within the mediastinum and lungs. Finally, CT may also be helpful for surgical planning, when appropriate.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-244). BPL reports other from Elsevier, outside the submitted work. JB Ackman reports other from Elsevier, outside the submitted work. JAS reports other from Elsevier, outside the submitted work. SRD reports other from Merck, other from Pfizer, other from Bristol Mayer Squibb, other from Novartis, other from Roche, other from Polaris, other from Cascadian, other from Abbvie, other from Gradalis, other from Clinical Bay, other from Zai laboratories, other from Siemens Medical Solutions, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review board approved this study, with informed consent waived due to its retrospective nature (IRB# 2018P003136).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Qureshi YA, Muntzer Mughal M, Markar SR, et al. The surgical management of non-malignant aerodigestive fistula. J Cardiothorac Surg 2018;13:113. [Crossref] [PubMed]
- Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg 2008;34:1103-7. [Crossref] [PubMed]
- Rodriguez AN, Diaz-Jimenez JP. Malignant respiratory-digestive fistulas. Curr Opin Pulm Med 2010;16:329-33. [Crossref] [PubMed]
- Cherveniakov A, Tzekov C, Grigorov GE, et al. Acquired benign esophago-airway fistulas. Eur J Cardiothorac Surg 1996;10:713-6. [Crossref] [PubMed]
- Mangi AA, Gaissert HA, Wright CD, et al. Benign broncho-esophageal fistula in the adult. Ann Thorac Surg 2002;73:911-5. [Crossref] [PubMed]
- Santosham R. Management of Acquired Benign Tracheoesophageal Fistulae. Thorac Surg Clin 2018;28:385-92. [Crossref] [PubMed]
- Shen KR. Management of acquired nonmalignant tracheoesophageal fistula: Surgical pearls. J Thorac Cardiovasc Surg 2017;154:e123. [Crossref] [PubMed]
- Bibas BJ, Guerreiro Cardoso PF, Minamoto H, et al. Surgical Management of Benign Acquired Tracheoesophageal Fistulas: A Ten-Year Experience. Ann Thorac Surg 2016;102:1081-7. [Crossref] [PubMed]
- Downey P, Middlesworth W, Bacchetta M, et al. Recurrent and congenital tracheoesophageal fistula in adults. Eur J Cardiothorac Surg 2017;52:1218-22. [Crossref] [PubMed]
- El-Essawy MT. Adult congenital tracheo-esophageal fistula with esophageal dysmotility and bronchiectasis. Saudi Med J 2011;32:305-7. [PubMed]
- Yasuda M, Hanagiri T, Ichiki Y, et al. Congenital tracheoesophageal fistula in an elderly patient with thoracic empyema. Gen Thorac Cardiovasc Surg 2009;57:622-4. [Crossref] [PubMed]
- Berkmen YM, Auh YH. CT diagnosis of acquired tracheoesophageal fistula in adults. J Comput Assist Tomogr 1985;9:302-4. [Crossref] [PubMed]
- Chaky DM, Escamilla C, Sheridan PH, et al. Adult Bronchoesophageal Fistula Diagnosed on Computed Tomography. Radiol Case Rep 2015;3:126. [Crossref] [PubMed]
- Lawrence DA, Branson B, Oliva I, et al. MDCT of the central airways: Anatomy and pathology [Internet]. [Accessed: 2020 Mar 15]. Available online: https://www.appliedradiology.com/articles/mdct-of-the-central-airways-anatomy-and-pathology
- Dionigi G, Rovera F, Boni L, et al. Cancer of the esophagus: the value of preoperative patient assessment. Expert Rev Anticancer Ther 2006;6:581-93. [Crossref] [PubMed]
- Smulewicz JJ, Guerrero LE, Washington D, et al. Emergency computerized tomography of tracheoesophageal fistula in lung adenocarcinoma. J Natl Med Assoc 1988;80:817-8, 821. [PubMed]
- Nagata K, Kamio Y, Ichikawa T, et al. Congenital tracheoesophageal fistula successfully diagnosed by CT esophagography. World J Gastroenterol 2006;12:1476-8. [Crossref] [PubMed]
- Leeds WM, Morley TF, Zappasodi SJ, et al. Computed tomography for diagnosis of tracheoesophageal fistula. Crit Care Med 1986;14:591-2. [Crossref] [PubMed]
- Al Harakeh H, Tulimat T, Sfeir P, et al. Penetrating shrapnel injury to the chest presenting as a delayed tracheoesophageal fistula (TEF). A case report. Trauma Case Rep 2018;17:5-8. [Crossref] [PubMed]
- Dogan BE, Fitoz S, Atasoy C, et al. Tracheoesophageal fistula: demonstration of recurrence by three-dimensional computed tomography. Curr Probl Diagn Radiol 2005;34:167-9. [Crossref] [PubMed]
- Ratan SK, Varshney A, Mullick S, et al. Evaluation of neonates with esophageal atresia using chest CT scan. Pediatr Surg Int 2004;20:757-61. [Crossref] [PubMed]
- Colleran GC, Ryan CE, Lee EY, et al. Computed tomography and upper gastrointestinal series findings of esophageal bronchi in infants. Pediatr Radiol 2017;47:154-60. [Crossref] [PubMed]
- Garge S, Rao KLN, Bawa M. The role of preoperative CT scan in patients with tracheoesophageal fistula: a review. J Pediatr Surg 2013;48:1966-71. [Crossref] [PubMed]
- Mahalik SK, Sodhi KS, Narasimhan KL, et al. Role of preoperative 3D CT reconstruction for evaluation of patients with esophageal atresia and tracheoesophageal fistula. Pediatr Surg Int 2012;28:961-6. [Crossref] [PubMed]
- Wychulis AR, Ellis FH, Andersen HA. Acquired nonmalignant esophagotracheobronchial fistula. Report of 36 cases. JAMA 1966;196:117-22. [Crossref] [PubMed]
- Expert Panel on Gastrointestinal Imaging, Levy AD, Carucci LR, et al. ACR Appropriateness Criteria® Dysphagia. J Am Coll Radiol 2019;16:S104-15. [Crossref] [PubMed]
- Roh S, Iannettoni MD, Keech JC, et al. Role of Barium Swallow in Diagnosing Clinically Significant Anastomotic Leak following Esophagectomy. Korean J Thorac Cardiovasc Surg 2016;49:99-106. [Crossref] [PubMed]
- Lantos JE, Levine MS, Rubesin SE, et al. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J Thorac Imaging 2013;28:121-8. [Crossref] [PubMed]
- Paraschiv M. Tracheoesophageal fistula - a complication of prolonged tracheal intubation. J Med Life 2014;7:516-21. [PubMed]
- Payne DK, Anderson WM, Romero MD, et al. Tracheoesophageal fistula formation in intubated patients. Risk factors and treatment with high-frequency jet ventilation. Chest 1990;98:161-4. [Crossref] [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [Crossref] [PubMed]


