Evaluation of the efficacy and safety of anti-PD-1 and anti-PD-L1 antibody in the treatment of non-small cell lung cancer (NSCLC): a meta-analysis
Introduction
Radiotherapy, chemotherapy and targeted agents have been widely accepted as standard treatments for patients with non-small cell lung cancer (NSCLC) (1,2). Despite new treatment options over the last decade, progress in lung cancer treatment in the broader population has reached a plateau, with limited additional benefits for patients lacking a driver mutation or translocation (3-6).
Under the particular circumstance, immune checkpoint inhibition therapy came into our sight as a new optional therapeutic approach for patients insensitive to those standard treatments, which acts directly on the tumor cells by restoring the immune system’s capacity to recognize and eradicate tumors (7). Of many molecularly defined checkpoint ligands and receptors, only blockers to cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1) and PD-1 ligand 1 (PD-L1) have been tested clinically to date (8,9).
Unlike melanoma, ipilimumab, an anti-CTLA-4 blocking mAb, doesn’t provide significant benefits in NSCLC patients as a single agent (10). Thus, PD-1 pathway has been extensively investigated. PD-1 predominantly regulates later effector T-cell activity within tissues and tumors. Tumor cells can suppress the activity of T cells in tissues and the tumor microenvironment by binding to PD-L1 on the T cells with its upregulating PD-L1 (11). PD-1 is also induced on other activated non-T-lymphocyte subsets, including B cells and NK cells, which may be inhibited by tumors expressing PD-L1 or PD-L2, as well (12). Several antibodies have been developed to either block the PD-1 or its ligand PD-L1. PD-1 inhibitors that are currently under development include Nivolumab (BMS-936558), the most clinically studied anti-PD-1 agent, and Pembrolizumab (MK-3475). As for PD-L1 inhibitors, BMS-936559, MPDL3280A and MEDI4736 have been in evaluation (13,14).
So far, a series of phase I trials on PD-1/PD-L1 antibodies for NSCLC have been completed, without final reports of results from phase II studies (15). Considering the limited sample size of these phase I studies, it is important and interested to conduct a timely summarization. Thus, we sought to perform a meta-analysis incorporating all available evidences to evaluate the efficacy and safety of PD-1 or PD-L1 inhibition therapy (14-28).
Material and methods
Literature search
All relevant articles were retrieved by searching PubMed, Embase and the Central Register of Controlled Trials of the Cochrane Library using a combination of the terms “PD-1”, “PD-L1”, “B7-1”, “Lung”, “non-small-cell lung cancer”, “NSCLC” and “anti-PD-1”. An additional search through Google Scholar and a manual search through reference lists of relevant reviews were additionally performed. Two authors (M Jia and W Feng) carried out the search independently. No restriction by language or year was set in the search.
Inclusion and exclusion criteria
Eligible studies should meet the following criteria: (I) clinical trials which investigate or report NSCLC patients with PD-1 antibody or PD-L1 antibody treatment; (II) the primary outcome was available. Studies failed to meet the inclusion criteria will be excluded.
Outcomes measures, data extraction and quality assessment
The primary outcome for this meta-analysis was objective respond rate (ORR). Data of ORR were extracted from the primary outcomes of each article. Other outcomes were rate of Grade 3-4 adverse effects (AEs) and rate of drug-related death. The data collection and assessment of methodological quality followed the Quorum and the Cochrane Collaboration guidelines (http://www.cochrane.de). The data on lead author, drug, patient status, study category, exon of epidermal growth factor receptor (EGFR) mutation, smoking status, ORR, disease control rate (DCR), and progression-free survival (PFS) were extracted by two investigators (W Feng and W Liang) independently. Three reviewers (S Kang, Y Zhang and J Shen) used a modified Newcastle-Ottawa scale to assess all the prospective and retrospective studies. Discrepancies were discussed by all investigators to reach consensus.
Statistical analysis
Odds ratios (ORs) for dichotomous data (ORR, rate of Grade 3-4 AEs and rate of drug-related death) with 95% CI were pooled. Heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). Random-effects model was employed in case of potential heterogeneity and to avoid underestimation of standard errors of pooled estimates in our meta-analyses. All calculations were performed using Meta-Analysis Beta 3.13 (Tufts medical center, Boston, Massachusetts, USA) and STATA 11.0 (StataA Corp, College Station, TX, USA). Subgroup analysis was conducted according to agent dosage and pathological type respectively. An OR value greater than 1 reflected a better ORR in higher dosage group. All CIs had two-sided probability coverage of 95%. A statistical test with P value less than 0.05 was considered as significant.
Publication bias
An extensive search strategy was made to minimize the potential for publication bias. Graphical funnel plots were generated to visually assess a publication bias. The statistical methods to detect funnel plot asymmetry were the rank correlation test of Begg and Mazumdar and the regression asymmetry test of Egger (29,30).
Results
Eligible studies
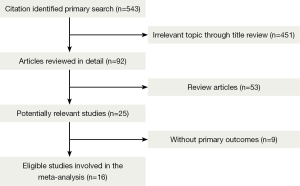
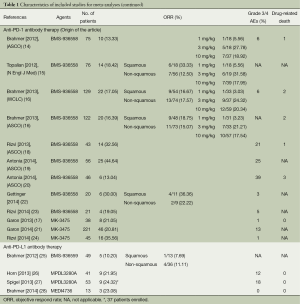
A total of 543 records were identified according to the search strategy and finally we enrolled 12 studies featured on PD-1 inhibition therapy involving 892 NSCLC patients and four studies about PD-L1 inhibition therapy involving 156 NSCLC patients. Figure 1 summarized the flow chart. Data of rate of Grade 3-4 AEs were not available in three studies and drug-related death rate were not available in seven studies, so that they were excluded in related analysis. Table 1 summarized the characteristics of involved studies for meta-analysis.
Full table
Meta-analyses of PD-1 and PD-L1 inhibition therapy in terms of ORR, rate of Grade 3-4 AEs and rate of drug-related death
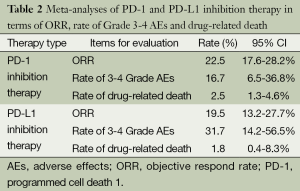
In overall, ORR in the whole population with PD-1 blockage treatment is 22.5% (95% CI: 17.6% to 28.2%). Additionally, the rate of Grade 3-4 AEs is 16.7% (95% CI: 6.5% to 36.8%) and drug-related death rate is 2.5% (95% CI: 1.3% to 4.6%).
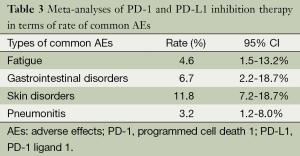
As for patients with PD-L1 inhibition therapy, an overall ORR is 19.5% (95% CI: 13.2% to 27.7%). A higher rate of Grade 3-4 AEs (31.7%, 95% CI: 14.2% to 56.5%) is observed with a lower drug-related death rate (1.8%, 95% CI: 0.4% to 8.3%, P<0.01) (Table 2). Rates of common AEs of anti-PD-1 agents were analyzed, including 4.6% fatigue (95% CI: 1.5% to 13.2%), 6.7% gastrointestinal disorders (95% CI: 2.2% to 18.7%), 11.8% skin disorders (95% CI: 7.2% to 18.7%), and 3.2% pneumonitis (95% CI: 1.2% to 8%). No Grade 3 or higher pneumonitis was seen in patients with anti-PD-L1 agents (Table 3).
Full table
Full table
Subgroup analyses, sensitivity analyses and publication bias
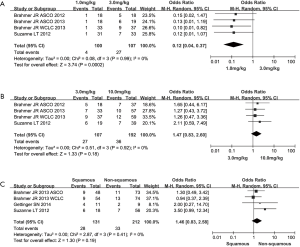
When stratifying patients according to agent dose of anti-PD-1 agents, we observed that greater ORR was presented in the median-dose cohort (3 mg/kg) than that of both low-dose (1 mg/kg) and high-dose (10 mg/kg) cohort (low-dose vs. median-dose: OR =0.12, 95% CI: 0.04 to 0.37, P=0.0002; median-dose vs. high-dose: OR =1.47, 95% CI: 0.83 to 2.60, P=0.18) (Figure 2A,B). In terms of pathological type, a trend of higher ORR were found in patients with squamous carcinoma (squamous vs. non-squamous: OR =1.46, 95% CI: 0.83 to 2.58, P=0.18) (Figure 2C). With regard to the publication bias, no significant bias was observed for all outcomes through both Begg’s test and Egger’s test (P>0.05).
Discussion
For NSCLC patients, the efficacy and safety of PD-1 and PD-L1 inhibition agents are still under initial investigation. A meta-analysis incorporating all available data from correlative studies is a good way to examine the current evidence. We conducted this study and found that both PD-1 and PD-L1 blockers showed durable outcome of ORR with a tolerable AEs and drug-related death rate in NSCLC patients. Additionally, a relatively optimal dosage and promising benefit population was explored in our work.
We found that both anti-PD-1 and anti-PD-L1 therapy showed a relatively lower ORR, higher 3-4 Grade AEs rate and higher drug-related death, compared to chemotherapy or TKIs (31-33). A possible explanation may be that the benefit population wasn’t screened in these clinical trials. In addition, duration of response/stable disease, as well as long-term survival outcomes are not available at present. This implies that further exploration on recognition of advantage group for PD-1/PD-L1 blockers is needed. Besides, although the maximum safe dose wasn’t reached, a dose of 3 mg/kg for PD-1 blockers was believed to be of the most efficacy and the less side effects among three types of dosage in our subgroup analysis of dose escalation.
Immune checkpoint inhibitors have shown promising activity with manageable toxicity in patients with NSCLC and may have an important role in the future treatment spectrum with broad patient applicability. Because of limited time and data, a complete evaluation of the anti-PD-1 and anti-PD-L1 agents is not yet possible. A preemptive approach to managing irAEs and sharing experience regarding understanding the clinical response patterns with immunotherapy with the melanoma community will help facilitate the introduction of this treatment approach to the lung cancer setting.
Acknowledgements
Authors’ contributions: Minghan Jia, Jianxing He and Wenhua Liang conceived and designed the experiments. Minghan Jia, Weijiao Feng and Shiyang Kang carried out the search. Jiaxi He, Long Jiang and Wei Wang extracted the data. Zhihua Guo, Guilin Peng and Gang Chen assessed the quality of included studies. Yaxiong Zhang and Shiyang Kang conducted the statistical analyses. Minghan Jia, Weijiao Feng and Wenhua Liang wrote the manuscript. All of the authors conducted a primary revision.
Disclosure: The authors declare no conflict of interest.
References
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [PubMed]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267-73. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- D’Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v116-9. [PubMed]
- Cufer T, Ovcaricek T, O'Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 2013;49:1216-25. [PubMed]
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297-339. [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [PubMed]
- Lu J, Lee-Gabel L, Nadeau MC, et al. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract. 2014; [PubMed]
- Zatloukal P, Heo DS, Park K, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675, 206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2009;27:abstr 8071.
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [PubMed]
- Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013;19:4917-24. [PubMed]
- Anti-PDL1 (MPDL3280A). Genentech Inc. Available online: http://www.biooncology.com/pipeline-molecules/anti-pdl1. Accessed December 5, 2013.
- Brahmer JR, Horn L, Antonia SJ, et al. Clinical activity and safety of anti-PD1 (BMS-936558, MDX-1106) in patients with advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7509.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Brahmer JR, Horn L, Antonia SJ, et al. Survival and long-term follow-up of the phase I trial of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with previously treated advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8030.
- Garon EB, Balmanoukian A, Hamid O, et al. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC). Presented at: 15th World Conference on Lung Cancer; October 27-30, 2013; Sydney, Australia. Abstract MO18.02.
- Rizvi NA, Antonia SJ, Laura Q.M. Chow, et al. A phase I study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) in chemotherapy-naive non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 2013;31:abstr 8072.
- Antonia SJ, Brahmer JR, Gettinger SN, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8113.
- Antonia SJ, Gettinger SN, Laura Q.M. Chow, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 2014;32:abstr 8023.
- Garon EB, Leighl NB, Rizvi NA, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8020.
- Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 2014;32:abstr 8024.
- Rizvi NA, Laura Q.M. Chow, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol 2014;32:abstr 8022.
- Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8007.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Horn L, Herbst RS, Spiegel D, et al. An analysis of the relationship of clinical activity to baseline EGFR status, PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1). IASLC 14th World Conference on Lung Cancer; 2011 Jul 2-5; Amsterdam, the Netherlands. MO18.01.
- Spigel DR, Gettinger SN, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8008.
- Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32:abstr 8021.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Rossi A, Pasquale R, Esposito C, et al. Should epidermal growth factor receptor tyrosine kinase inhibitors be considered ideal drugs for the treatment of selected advanced non-small cell lung cancer patients? Cancer Treat Rev 2013;39:489-97. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]