Serial disseminated intravascular coagulation score with neuron specific enolase predicts the mortality of cardiac arrest—a pilot study
Introduction
Out-of-hospital cardiac arrest (OHCA) patients have high mortality worldwide, even in those in whom a successful return of spontaneous circulation (ROSC) had been obtained, in-hospital survival rate remains considerably low (1,2). The underlying mechanisms for high mortality and neurologic dysfunction of patients achieved ROSC has been attributed to post-cardiac arrest syndrome (PCAS) (3). The 4 key components of PCAS are post-cardiac arrest (CA) brain injury, post-CA myocardial dysfunction, systemic ischemia/reperfusion response and persistent precipitating pathology. The whole-body ischemia/reperfusion of CA with associated oxygen debt causes generalized activation of immunological and coagulation pathways, increasing the risk of multiple organ failure and infection. Coagulation dysfunction is considered one pathophysiology leading to disseminated intravascular coagulation (DIC), which clinically manifests as obstruction of microcirculation and multiple organ dysfunction (4). As a result, intravascular thrombosis causing cerebral microvascular occlusion produces the no-reflow phenomenon. Therefore, DIC is deeply involved in the pathophysiology of post-CA brain injury, which is well-recognized as a cause of early mortality after out-of-hospital (OHCA) (1). Recent studies suggest the activity of coagulation and fibrinolysis can serve as a prognostic factor for mortality and poor neurologic outcome (5-8).
However, studies suggest that treatment after CA, mainly sedation and targeted temperature management (TTM), may substantially delay awakening and alter the accuracy of prognostication in this setting (9-11). Traditional prognostic methods are influenced by sedation and TTM, causing biases in prognostication. Thus, predicting survival rates and neurological outcome after CA remains a major challenge for physicians. Consequently, outcome prediction in comatose patients after CA has evolved toward a multimodal approach recommended by researchers (12,13). Because hypercoagulability is deeply involved in the pathophysiology of post-CA brain injury, using coagulation parameters is a promising way to facilitate predicting outcome.
Biomarkers of brain damage after CA, particularly neuron-specific enolase (NSE), have been demonstrated as markers for CA (13). It has been proved useful tool for prognostication following OHCA (14-16), which is suggested as a part of multimodal prognostication strategy by current guidelines (17,18). In-depth research is needed to evaluate the diagnostic value of NSE.
In light of recent research progress in neurologic biomarker after CA, we used a multimodal approach combining serial DIC scores and NSE for predicting discharge mortality for CA patients receive ROSC. The study aimed to improve prognostic value in OHCA patients by combining serial DIC scores and NSE.
Methods
This single-center study was performed analyzing data between July 2014 and December 2016 in Peking University Third Hospital (Beijing, China). The institutional Ethics Committee approved the study and issued a waiver of consent since all examinations were part of standard patient care. The IRB approval number is 2013058. It should be noticed that the manuscript is a sub-study of the whole research regarding CA named “Cross-sectional study in mild hypothermia therapy and standard procedure development for CA patients in Beijing”. The study is conformed to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
Study setting
The study center was a 1,755 clinic beds tertiary academic hospital with approximately annual 4 million outpatient and 30 thousand emergency visits. Beijing Emergency Medical Service supported by Beijing Emergency Medical Centre and Beijing Red Cross Emergency Rescue Centre covers the entire area (19). Treatment in the field was in accordance with the European Resuscitation Council and the American Heart Association guidelines for basic and advanced cardiac life support and post resuscitation care (20). OHCA patients were enrolled regardless of initial heart rhythms. All CA patients received ROSC took serial blood sampling immediately since Emergency department arrival as there was standard blood sampling protocol for OHCA patients in our institution. All the patients with ROSC received TTM as a part of conventional treatment.
Participants and data collection
The study population was all emergency department OHCA patients who achieved ROSC. Demographic and clinical information were retrospectively collected in the information system. Researchers are physicians well-trained in resuscitation. Before the initiation of study, repeated group meetings designed a standard searching strategy applied by researchers. The search term is “Out-of-hospital cardiac arrest” to retrieve all the cases. The name and identify number of the patient was deleted by the final analysis. All the researchers have access to the database without blindness. Laboratory results that missing at random (less than 20%) were interpolated using multiple imputation. Patients with missing value regarding baseline characteristics or outcome were excluded. Serial blood samples were routinely collected from admission to 1 week after CA. Coagulation tests and NSE levels were extracted from laboratory information system. The patients aged 18 years or older were included. Patients were excluded if: (I) pre-arrest cognitive impairment; (II) existing terminal illness; (III) missing data regarding baseline characteristics or outcome.
Outcome was death before hospital discharge. Neurologic outcome was demonstrated using cerebral performance category (CPC) score. A poor neurologic outcome was defined as a CPC-score of 3–5 equivalent to severe disability, coma or death (21). The DIC score was calculated using the methods suggested by the International Society of Thrombosis and Haemostasis (22). It should be mentioned that none of the patents underwent withdrawal of life sustaining therapy during their stay. The decision of withdrawal life sustaining therapy was unclear after discharge.
Statistical analysis
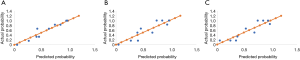
All continuous variables are described as the mean ± SD (normal distribution) or median ± quartile (abnormal distribution). Kolmogorov Smirnov test was conducted to test normal distribution and Homogeneity of variance test was conducted. Categorical variables are expressed as percentages. Frequencies were compared using either the chi-square or Fischer’s exact test as appropriate. Mann-Whitney U test was used to test differences between survival and death groups when showed non-normal distributions. To evaluate the prognostic value of the DIC score and NSE, receiver operating characteristic (ROC) analysis was performed. Binary logistic regression was adopted to get the combined probability of DIC score and NSE. For further validation of the combining model, Hosmer-Lemeshow test was used for internal validation of predictive models. To visualize the results of Hosmer-Lemeshow test, calibration curves is drawn. All statistical analyses were performed using SPSS version 22.0 (IBM Inc., Armonk, NY, USA). The reported P values are two-sided. A P value <0.05 was considered to be statistically significant.
Patient and public involvement
It is a retrospective study. Development of the research question and outcome measures was not influenced by patients’ priorities, experience, and preferences. Patients and public were not involved in the process of the study. The results will not be disseminated to study participants.
Results
Baseline characteristics
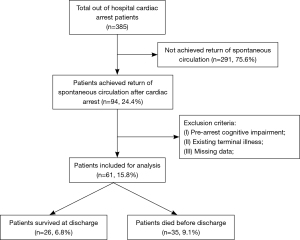
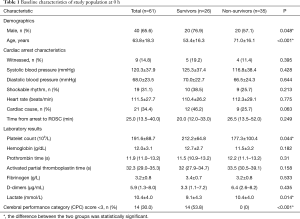
As shown in Figure 1, 61 patients were included in the final analysis. Of the included patients, 65.6% were male, and the mean age was 63.8±18.3 years. Table 1 details the prehospital characteristics and laboratory results at 0 h. Patients characteristic of population were divided according to survival status. Significant difference was observed in age, platelet count and lactate. Non-survivors were more likely to be older (P<0.001), with decreased platelet count (P<0.05) and increased lactate level (P<0.05).
Full table
DIC scores and NSE values in both groups
DIC scores were calculated according to coagulation function at 0, 4, 12, 24, 48, 72 h and 1 week. The scores were significantly higher at 24 and 48 h after ROSC in the non-survivors (shown in Figure 2).

Figure 2 shows NSE values over the first week after ROSC. Median values are described for survivors versus non-survivors at 0, 12, 24, 48, 72 h and 1 week respectively. NSE values were significantly higher in the non-survivors versus the survivor’s group at all time point except the 0-h group.
Evaluation of the prognostic value of parameters for discharge mortality
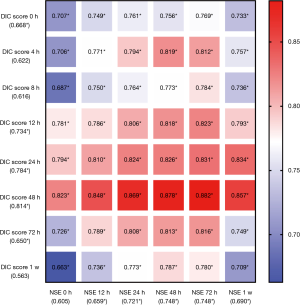
Predictive performances for discharge mortality were assessed using ROC curve. Figure 3 demonstrates the area under curve (AUC) describing the diagnostic accuracy of DIC score combining NSE at all time points. The AUCs for each single test are illustrated as well (Table 2). Finally, the best three predictive combinations were separated. The AUCs are 0.869 (95% CI, 0.781–0.956), 0.878 (95% CI, 0.791–0.965) and 0.882 (95% CI, 0.792–0.972) for DIC score at 48 h combining NSE at 24 h, DIC score at 48 h combining NSE at 48 h and DIC score at 48 h combining NSE at 72 h respectively (Figure 4). Table 3 demonstrates the sensitivity and specificity by maximizing the Youden index for predicting non-survival, which is 0.59, 0.62 and 0.65 for each group. Positive predictive value and false predictive value are shown for each group as well. The Kaplan-Meier survival curve for the analysis set is shown in Figure 5.
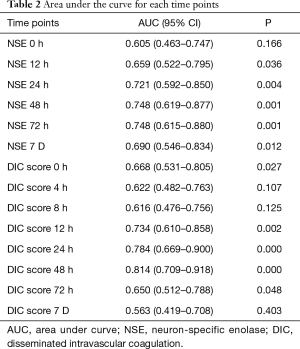
Full table
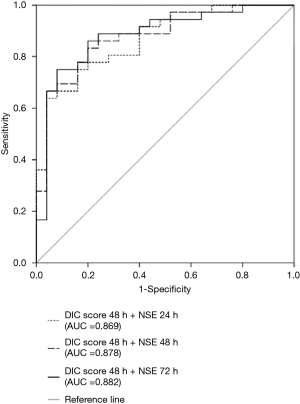
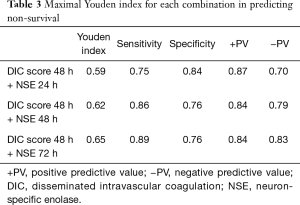
Full table
Further test for internal validation of predictive models was conducted. Figure 6 demonstrates calibration curves for the selected three predictive combinations. Significance of Hosmer-Lemeshow test is 0.488, 0.324, 0.011 for DIC score at 48 h combining NSE at 24 h, DIC score at 48 h combining NSE at 48 h and DIC score at 48 h combining NSE at 72 h respectively.
Discussion
In the present study, we demonstrated that combining the DIC score with NSE could have favorable predictive value with the AUCs over 0.8. To our knowledge, this is the first reporting the predictive value combining serial DIC score and NSE. Our finding provides a novel approach that can be useful for the prediction of death probability. Our study showed patients with higher DIC scores is associated with a poor prognosis at 24 and 48 h after ROSC. Interestingly, NSE proved to be distinguished between groups around the same time points. The NSE Levels continued to increase in the first 72 h in non-survivors. While NSE levels in survivors tended to decrease after 48 h. The NSE peak time was similar with other studies (14,16). In the further analysis, coagulation/anticoagulation and fibrinolysis/anti-fibrinolysis systems are activated in patients who undergo cardiopulmonary resuscitation (3). The recognition, measurement, and treatment of coagulation dysregulation seen during and after CA represents a significant impediment to improving survival from CA (23). Deng et al. measured serum D-dimer as a useful indicator of immediate mortality in patients with in-hospital CA (24). Kim et al. (25) found that an increased initial DIC score in OHCA patients was an independent predictor for poor outcomes and early mortality risk. Several studies have shown that various coagulofibrinolytic markers are associated with mortality in OHCA patients (26,27). Wada et al. demonstrated DIC patients more frequently developed system inflammatory reaction syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), followed by worse outcomes than non-DIC patients (5). Accompanying high mortality has been explained by that intravascular fibrin formation and microthromboses are distributed throughout the entire microcirculation (28).
Meanwhile, NSE proves to be a robust marker in the present study. The predictive performance in our cohort implies DIC score and NSE are independently related to outcome, suggesting that they provide complementary information. DIC worsen microcirculation of brain with the elevating NSE resulted from ischemia and hypoxia. Consequently, DIC score and NSE could reflect severity of neurologic defect comprehensively.
Previous multimodal approach studies offered various combinations. Youn et al. suggested combining neurologic examination and imaging (29). In comparison, Oddo et al. yielded good predictive performance combining clinical examination, electroencephalography reactivity and serum NSE (30). Our model has advantages over the former ones since both DIC score and NSE are widely available in the clinical settings. They are more accessible than imaging data. The study did not draw a conclusion about the best timing for this combination and the best cut-off value. High proportion of CA patients died shortly after onset, for whom predicting outcome is less meaningful. Our research result demonstrates that laboratory result from 24 to 72 h show excellent predictive value, which is a critical time for prognosis evaluation. Our simple size is relatively small and the laboratory method of NSE is discrepant across different medical institution. External validity should be considered in the further studies.
Conclusions
Increased DIC score or NSE levels is associated with higher discharge mortality in CA patients. Combining serial DIC score and NSE improve the prognostic value of single test. Further studies with more patients are needed to validate the model.
Acknowledgments
We acknowledge the colleagues of emergency department at the Peking University Third Hospital.
Funding: Peking University Third Hospital Clinical Key Cultivation Exploration Project “Comparison of application in extracorporeal cardiopulmonary resuscitation and traditional cardiopulmonary in middle-young cardiac arrest patients, BYSY2016005”; Capital Characteristic Clinic Project “Monitoring of hypothermia treatment after resuscitation and development and application of the standard procedure for hypothermia treatment of cardiac arrest patients in Beijing area”.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037//jtd-20-580
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-580). The authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Peking University Third Hospital Ethics Committee approved the study (No. 2013058) and issued a waiver of consent since all examinations were part of standard patient care. It should be noticed that the manuscript is a sub-study of the whole research regarding cardiac arrest named “Cross-sectional study in mild hypothermia therapy and standard procedure development for cardiac arrest patients in Beijing”. The study is conformed to the provisions of the Declaration of Helsinki (as revised in 2013),
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lemiale V, Dumas F, Mongardon N, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med 2013;39:1972-80. [Crossref] [PubMed]
- Wissenberg M, Lippert FK, Folke F, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA 2013;310:1377-84. [Crossref] [PubMed]
- Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008;79:350-79. [Crossref] [PubMed]
- Wada T. Coagulofibrinolytic Changes in Patients with Post-cardiac Arrest Syndrome. Front Med (Lausanne) 2017;4:156. [Crossref] [PubMed]
- Wada T, Gando S, Ono Y, et al. Disseminated intravascular coagulation with the fibrinolytic phenotype predicts the outcome of patients with out-of-hospital cardiac arrest. Thromb J 2016;14:43. [Crossref] [PubMed]
- Viersen V, Greuters S, Korfage A, et al. Hyperfibrinolysis in out of hospital cardiac arrest is associated with markers of hypoperfusion. Resuscitation 2012;83:1451-5. [Crossref] [PubMed]
- Schwameis M, Buchtele N, Schober A, et al. Prognosis of overt disseminated intravascular coagulation in patients admitted to a medical emergency department. Eur J Emerg Med 2017;24:340-6. [Crossref] [PubMed]
- Lee DH, Lee BK, Jeung KW, et al. Disseminated intravascular coagulation is associated with the neurologic outcome of cardiac arrest survivors. Am J Emerg Med 2017;35:1617-23. [Crossref] [PubMed]
- Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia*. Crit Care Med 2014;42:2493-9. [Crossref] [PubMed]
- Paul M, Bougouin W, Geri G, et al. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med 2016;42:1128-36. [Crossref] [PubMed]
- Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care 2011;15:113-9. [Crossref] [PubMed]
- Cronberg T, Brizzi M, Liedholm LJ, et al. Neurological prognostication after cardiac arrest—Recommendations from the Swedish Resuscitation Council. Resuscitation 2013;84:867-72. [Crossref] [PubMed]
- Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: An advisory statement from the European Resuscitation Council and the European Society of Intensive Care Med. Intensive Care Med 2014;40:1816-31. [Crossref] [PubMed]
- Gillick K, Rooney K. Serial NSE measurement identifies non-survivors following out of hospital cardiac arrest. Resuscitation 2018;128:24-30. [Crossref] [PubMed]
- Wiberg S, Kjaergaard J, Kjærgaard B, et al. The biomarkers neuron-specific enolase and S100b measured the day following admission for severe accidental hypothermia have high predictive values for poor outcome. Resuscitation 2017;121:49. [Crossref] [PubMed]
- Stammet P, Collignon O, Hassager C, et al. Neuron-Specific Enolase as a Predictor of Death or Poor Neurological Outcome After Out-of-Hospital Cardiac Arrest and Targeted Temperature Management at 33°C and 36°C. J Am Coll Cardiol 2015;65:2104-14. Erratum in: J Am Coll Cardiol 2015;66:983. Correction. J Am Coll Cardiol 2017;69:2104. [Crossref] [PubMed]
- N Correction to. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2017;136:e197. [PubMed]
- Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Med 2015 guidelines for post-resuscitation care. Intensive Care Med 2015;41:2039-56. [Crossref] [PubMed]
- Shao F, Li CS, Liang LR, et al. Outcome of out-of-hospital cardiac arrests in Beijing, China. Resuscitation 2014;85:1411-7. [Crossref] [PubMed]
- Hazinski MF, Field JM. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science. Pediatrics 2010;126:1361-99. [Crossref]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480-4. [Crossref] [PubMed]
- Taylor FBJ, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86:1327-30. [Crossref] [PubMed]
- Kurz MC. Which comes first? The chicken or the egg: The association of d-dimer with return of spontaneous circulation following in-hospital cardiac arrest. Thromb Res 2016;143:159-60. [Crossref] [PubMed]
- Deng Y, He L, Yang J, et al. Serum D-dimer as an indicator of immediate mortality in patients with in-hospital cardiac arrest. Thromb Res 2016;143:161-5. [Crossref] [PubMed]
- Kim J, Kim K, Lee JH, et al. Prognostic implication of initial coagulopathy in out-of-hospital cardiac arrest. Resuscitation 2013;84:48-53. [Crossref] [PubMed]
- Ono Y, Hayakawa M, Maekawa K, et al. Fibrin/fibrinogen degradation products (FDP) at hospital admission predict neurological outcomes in out-of-hospital cardiac arrest patients. Resuscitation 2017;111:62-7. [Crossref] [PubMed]
- Szymanski FM, Karpinski G, Filipiak KJ, et al. Usefulness of the D-dimer concentration as a predictor of mortality in patients with out-of-hospital cardiac arrest. Am J Cardiol 2013;112:467-71. [Crossref] [PubMed]
- Bttiger BW, Motsch J, Bhrer H, et al. Activation of Blood Coagulation After Cardiac Arrest Is Not Balanced Adequately by Activation of Endogenous Fibrinolysis. Circulation 1995;92:2572-8. [Crossref] [PubMed]
- Youn CS, Callaway CW, Rittenberger JC. Combination of initial neurologic examination, quantitative brain imaging and electroencephalography to predict outcome after cardiac arrest. Resuscitation 2017;110:120-5. [Crossref] [PubMed]
- Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 2014;42:1340-7. [Crossref] [PubMed]