
Change in quality of life of stage IA lung cancer patients after sublobar resection and lobectomy
Introduction
Introduction of CT screening has increased the frequency of early stage lung cancer diagnoses, and thus also the number of long-term lung cancer survivors. Concurrently, treatment choices have also expanded to include important technologic advancements in surgery and radiotherapy and immunotherapy. To provide guidance to treating physicians, ongoing randomized trials are comparing alternative surgical approaches (1,2) and surgery with stereotactic radiotherapy (3-6) for non-small cell lung cancer (NSCLC). Prior to completion of these trials, and even upon their completion, treating physicians and their patients are faced with choosing a treatment that is personalized to the particular patient’s situation. Physicians are involving their patients in this decision-making process, as patient-centered elements must be considered prior to deciding on treatment.
In 2014, focus groups were held separately with thoracic surgeons and early stage surgical lung cancer survivors (7). The qualitative analysis of the focus groups suggested that surgeons prioritized clinical indicators to decide on the best surgical approach and the extent of surgical resection, whereas patients focused on the long-term consequences of their surgery and wanted to better understand their options and the resulting consequences on their survival and quality of life (QoL) after surgery. The patients expressed feelings of isolation, anxiety, and persistent and pervasive pain, previously not well recognized. These results underscore the need for increased focus on minimizing morbidity of treatment and maximizing QoL.
A systematic review found that, while a body of literature on QoL after treatment for NSCLC is emerging (8), few studies focused on early stage lung cancer (9-16). This review coupled with the results of the focus groups led us to an investigation of QoL scores, using Short Form 12, which had been collected on early stage lung cancer patients by the International Early Lung Cancer Action Program since 2001 (9,10). A comparison of pre- and post-surgical QoL scores of Stage IA NSCLC patients diagnosed by CT screening found a significant decrease in physical health scores from pre- to one year after surgery among lobectomy (L) patients but not among sublobar resection (SL) patients (9). When the authors examined the effect of two surgical approaches [video-assisted thoracoscopic surgery (VATS) vs. traditional open chest thoracotomy], they found no significant difference between the pre and post-surgical scores of overall physical health or mental health (10).
In 2016, a prospective cohort study of documented Stage I NSCLC patients receiving surgical, radiotherapy, or other treatment [the Initiative for Early Lung Cancer Research on Treatment (IELCART)] was started (17) in order to assess treatment differences in the course of clinical care by documenting the QoL measures before and after surgery to identify critical time points at which supportive interventions (e.g., additional social support, physical therapy) would be most helpful.
The current study aims to build on our previous work (10) by assessing the differential impact of the extent of surgical resection (SL vs. L) among VATS on QoL using longitudinal IELCART data in order to identify critical post-operative time points at which QoL significantly changes for each resection method.
Methods
We reviewed all patients enrolled in the prospective cohort study, IELCART since its start in 2016 who underwent VATS surgery for NSCLC, by the extent of surgery (SL or L). Sublobar resection included segmentectomy and wedge resection. We included all patients with a first primary NSCLC had pathologic Stage IA (T1a-1cN0M0) NSCLC (8th AJCC/UICC staging) (18) who did not receive adjuvant chemotherapy, radiation therapy, or subsequent surgery within 12 months of the initial surgery. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Mount Sinai Hospital (IRB# IF 2365016). At enrollment, HIPAA-compliant written informed consent was obtained from all participants.
Demographic, comorbidities, social support, pre-surgical CT findings, and post-surgical pathologic findings were documented. Clinical TNM staging was obtained for all participants, and pre-operative diagnosis, if available, was also recorded. Pre-surgical QoL scores were obtained by in-person interviews during the patient’s pre-surgical clinic visit. If that was not possible, telephone interviews were performed or the questionnaires were completed by the patients and returned via mail. Follow-up QoL scores were obtained at clinic visits within 4, 6, and 12 months after surgery.
Sociodemographic and medical characteristics
Prior to surgery, at the time of IELCART enrollment, baseline demographic data, smoking history, and 12 different comorbidities—presence of additional cancers, asthma, emphysema or chronic obstructive pulmonary disease, high blood pressure, high cholesterol, angioplasty or stent, myocardial infarction, stroke, peripheral vascular disease, liver disease, diabetes, and kidney disease—were collected on each patient. A comorbidity score was calculated by totaling the number of documented comorbidities for each patient, ranging from 0 to 12. Height and weight were documented and body mass index (BMI) was calculated in kilograms per meters squared (kg/m2); obesity was defined as a BMI ≥30 kg/m2.
Social support as perceived by each patient was documented at baseline enrollment using the Medical Outcomes Study Social Support Survey (MOS index) consisting of a 19-item questionnaire. Its five subscales are: emotional/informational support, tangible support, positive interaction, affection, and whether there is someone to help keep one’s mind off things (19). The overall MOS index score ranges from 0–100 with a higher score corresponding to better patient-perceived social support.
The tumor consistency on the pre-surgical CT scan was documented as solid, part-solid, or nonsolid (20). The post-surgical pathology results of the tumor cell-type and maximum diameter were documented for each patient.
Quality of life instruments
Physical Component Summary (PCS) and Mental Component Summary (MCS)
The 12-item Short Form (SF-12v2), a shorter version of SF-36v2 (21), is used to calculate two norm-based component scores, the PCS score and a MCS score. These two component scores are calculated using different standardized weighted summaries of eight domains of health: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health within the previous 4 weeks. For PCS, the four physical subscales have a more significant weight while for MCS, the four mental health subscales have more weight. The norm-based average for the United States population is a mean of 50 and standard deviation (SD) of 10 for both PCS and MCS; higher scores reflect better physical and mental health. A minimum of a 3-point difference has been suggested as a clinically important difference or change for both scores (21).
Functional Assessment of Cancer Therapy-Lung Cancer (FACT-LCS)
FACT-L is a multi-dimensional validated self-report instrument to document symptoms of different cancers (22). We used only the lung cancer subscale (LCS) which asks about symptoms of dyspnea, weight loss, mental clarity, coughing, appetite, tightness in the chest, and difficulty breathing within the previous 7 days. The FACT-LCS scores range from 0–28; a higher score means there are fewer symptoms. A 2- to 3-point difference has been suggested as being clinically meaningful (23).
The Patient Health Questionnaire-4 (PHQ-4)
The Patient Health Questionnaire-4 (PHQ-4) is composed of two subscores, GAD-2 and PHQ-2, each of which have two questions. The two-item GAD-2 Anxiety measure, drawn from the GAD-7 instrument (24), is obtained by adding the scores for two items. Possible scores range from 0–6, where lower scores correspond to less anxiety. A GAD-2 score of 3 or higher is the preferred cut-off for identifying patients with generalized anxiety disorder (25).
The two-item PHQ-2 Depression measure is drawn from the PHQ-9 instrument. The PHQ-2 score is obtained by adding the scores for the two items. Possible scores range from 0–6, where lower scores correspond to fewer symptoms of depression (24). A PHQ-2 score of 3 or higher is optimal cut-off score for clinical depression (26).
Statistical analyses
Results were summarized by means (SD) or medians (interquartile range, IQR) for continuous and ordinal data, and frequencies and percentages for categorical data. Comparison of SL and L patients, for continuous variables used two-sample t-tests for normally distributed variables, otherwise the Mann-U Whitney rank test was used. For categorical variables, Pearson’s χ2 test (or Fisher’s exact test where appropriate) was used.
The average score for each QoL measure (PCS, MCS, FACT-LCS, GAD-2, and PHQ-2) was calculated before treatment (T0), and for each postoperative month. The QoL postoperative scores were initially categorized into the following three time categories: (I) T1–T4 if the information was obtained within the first four postoperative months, (II) T5–T8 if obtained within 5 to 8 postoperative months, and (III) T9–T15 if obtained within 9 to 15 postoperative months, separately for SL and L (Table 1).
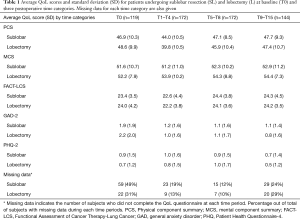
Full table
Analyses of missing data were performed separately for patients who had two or more consecutive missing QoL scores up to and including the 12-month evaluation (due to loss to follow-up), and for patients with intermittent missing QoL scores. Differences in the missing data patterns were assessed between surgical groups (27).
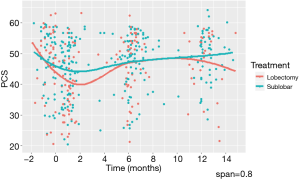
To explore critical time points when the trajectory of QoL scores shift, we first used nonparametric locally weighted smoothing (LOWESS) to fit a continuous curve across the time points when the data were collected. The LOWESS smoother was estimated by starting with the first time period, using 80% of the data closest to each time point (i.e., span =0.8) (Figure 1 provides an example for PCS). This plot allows for visualization of QoL trends and identification of times when an upward or downward shift of the QoL scores occurs (28). For PCS scores, the best interval knot for the change in the trajectory was at 2 months. LOWESS plots were made for the other QoL scores and these also pointed to an interval knot at 2 months.
To capture linear trends in QoL using time as a continuous variable, we developed a piecewise linear mixed effects model to estimate each of the QoL measures at baseline and throughout the postoperative period. Correlations between scores for each patient were accounted for by specifying a random intercept. This model posits that patients vary in baseline QoL scores and have different patient-specific trajectories during the post-operative 12-month time. Moreover, it allows us to characterize critical point(s) in time when the linear trajectory of the QoL measure changes (28-30). Using this model, the extent of surgery (SL or L) for any QoL measure can be estimated by averaging over patient-specific trajectories by surgical group membership. The model provides a more robust and flexible approach than a repeated measures analysis of variance model, because the usage of pre-defined time categories is not required and because the model better accommodates missing or mistimed QoL outcome measurements (29). We estimated two separate rates of change for each QoL measure; the first rate from time =0 (baseline) to 2 months after surgery, and the second rate from 2.1 to 12 months after surgery. In addition to including the piecewise time effects, the surgery (SL vs. L), and the interaction of time and extent of surgery, each QoL model included the effects of sex, race, post-secondary education, age, BMI, pack-years, the baseline MOS index, maximum pathologic tumor size, and comorbidity ordinal score as specified below:
E(Scoreij)=β1+ β2Timeij+β3(Timeij – t*)+ + β4Surgery_extenti+β5TimeijxSurgery_extenti+β6(Timeij–t*)+ xSurgery_extenti+β7Sexi+β8Racei+β9Collegei+β10Agei(centered)+β11BMIi(centered)+β12Packyrsi(centered)+β13MOSi(centered)+β14Tumor_sizei(centered)+β15Comorbidity_scorei(centered) [1]
with t*=2 months, (Timeij –t*)+ is equal to (Timeij –2) when Timeij >2 and equal to zero when Timeij ≤2. When expressed in terms of average response prior to or after t*=2 months, the final model for patients in the L group (Surgery_extenti=0), for example, would be:
E(Scoreij)=β1+ β2Timeij +.+βpXp, when Timeij ≤ t*; [2]
E(Scoreij)=(β1 - β3t*)+(β2 +β3)Timeij +.+βpXp, when Timeij >t* [3]
Thus the rate of change for time prior to 2 months was estimated by β2, and subsequent to 2 months the rate of change was estimated by (β2+β3). The fit of the final model for each of the QoL measures was assessed by evaluating the normality of the scaled residuals.
The final model(s) used covariate values (e.g., age, sex) to estimate the QoL score for each of the QoL measures at baseline (time =0) and at the postoperative times of 2, 6 and 12 months; for continuous covariates, the sample averages were used and the categorical covariates, the most representative subgroup was used. Each estimate was calculated separately for the SL and L patients. This model also allowed for assessing whether QoL measures differed among the covariates. All statistical analyses were performed using SAS Software version 9.4 and R version 3.5.2.
Results
Of the 201 participants included in this study, sublobar resection (SL) was performed more frequently than lobectomy (L) [127 (63.2%) vs. 74 (36.8%), P<0.001]. Table 2 shows that at baseline (T0), no significant differences were found between the SL and L patients as to age, sex, other sociodemographic or medical characteristics, except that the median tumor size on pathology was significantly smaller for SL than L patients (14 vs. 20 mm, P<0.001). Median MOS index scores on the baseline were not statistically significantly different.
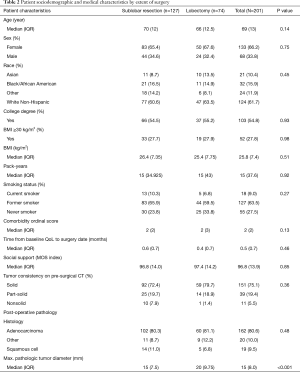
Full table
The average PCS score at baseline were 46.34 and 46.01, P=0.83, respectively, for SL and L patients (Table 3, Figure 2A). The average PCS scores for SL and L patients were below the average of the general population (mean ± SD: 50±10). For both SL and L patients, PCS scores decreased in the first two postoperative months, but the decrease was less severe for SL than for the L patients [−0.18 vs. −2.30, P=0.02]. PCS scores increased in the subsequent 10 postoperative months for both SL and L, but the monthly rate of increase for SL patients was slower than that for L patients [+0.29 vs. +0.74, P=0.06]. The average PCS score at 12 months was significantly higher than the average baseline PCS score for SL patients (48.88 vs. 46.34, difference =+2.55 or rounded to 3, P=0.02) and for L patients (48.79 vs. 46.01, difference =+2.78 or rounded to 3, P=0.048). Overall, the average physical health score for both SL and L patients started and remained below the general population average, both decreased significantly in the first two months after surgery, but significantly improved by 3 points by the end of the first postoperative year.
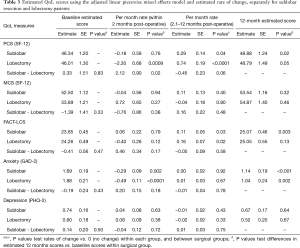
Full table
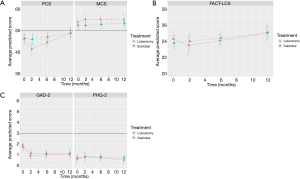
Average MCS scores at baseline for SL and L patients were 52.50 vs. 53.89, P=0.33. The average scores were slightly above that of the general population (Figure 2A). Within the first two postoperative months, MCS scores did not significantly change for either SL or L patients [−0.04 vs. +0.72, P=0.38] nor for the subsequent 10 postoperative months [+0.11 vs. −0.04, P=0.48]. In fact, the monthly rates of change were not significantly different for SL and L patients during the entire first post-operative year. At 12 months, the average MCS was not significantly higher than the average baseline MCS for SL patients (53.89 vs. 52.54, difference =1.35; rounded to 1, P=0.32) or for L patients (54.87 vs. 53.89, difference =+0.98; rounded to 1, P=0.46). Overall, the average mental health score for both SL and L patients was above the general population average prior to surgery, improved by one point at the end of the first operative year.
The average FACT-LCS symptom scores at baseline for SL and L patients were 23.85 vs. 24.26, P=0.47 (Figure 2B). FACT-LCS scores increased, showing an improvement in symptoms, and did not significantly differ between SL and L patients during the first two postoperative months [+0.06 vs. −0.40, P=0.17]. During the subsequent 10 postsurgical months, FACT-LCS scores continued to increase at similar rates for both SL and L patients [+0.11 vs. +0.16, P=0.58]. By 12 months, the average FACT-LCS score had significantly improved over the average baseline score for SL patients [25.08 vs. 23.85, difference =+1.23, P=0.003], but not for L patients [25.02 vs. 24.26, difference =+0.79, P=0.13].
The average GAD-2 anxiety scores at baseline for SL and L were 1.69 vs. 1.88, P=0.43 (Figure 2C). The scores significantly decreased for both SL and L patients during the first 2 postoperative months [−0.29, P=0.002 vs. −0.49, P<0.0001], showing a decrease in anxiety; the rates of decrease per month were similar for both SL and L patients (P=0.18). No further changes were observed for the next 10 postoperative months (0.00 vs. +0.01, P=0.78). The average GAD-2 scores at 12 months were significantly lower than the average baseline scores for SL patients [1.14 vs. 1.69, difference =−0.85 or rounded to 1, P=0.0002] and for L patients [1.04 vs. 1.88, difference =−0.58 or rounded to 1, P=0.001], showing that anxiety had decreased over the operative year for both SL and L patients.
The average PHQ-2 depression scores at baseline for SL and L were 0.74 and 0.60, P=0.50 (Figure 2C). The scores increased slightly for the first 2 postsurgical months for both SL and L patients [+0.04 vs. +0.08, P=0.72], suggesting increasing depression, and then improved over the remaining 10 postoperative months [−0.01 vs. −0.02, P=0.75]. The average PHQ-2 score at 12 months was lower than the average baseline scores for both SL and L patients [0.67 vs. 0.74, difference =−0.07, P=0.64] and [0.52 vs. 0.60, difference =−0.08, P=0.67]. Overall, depression scores had improved, but not significantly over the course of first postoperative year.
For all QoL measures, the average physical health, mental health, and lung cancer symptom scores were lower for women than for men. The FACT-LCS was significantly lower (P=0.003) by 1.58 points, rounded to 2 for women across all time points. The anxiety and depression scores were worse for women than for men, but only the anxiety GAD-2 score was significantly higher (P=0.04) by 0.39 points across all time points (P=0.04).
Older patients had significantly related to better mental health (MCS, P<0.001), and also lower anxiety (GAD-2, P<0.001) and depression (PHQ-4, P<0.001) scores. Having a post-secondary school degree was associated with significantly lower lung cancer symptom scores (FACT-LCS, P=0.009). More comorbidities was significantly related to lower physical health (PCS, P=0.01), increased symptoms (i.e., lower FACT-LCS, P=0.01), and higher depression symptoms (PHQ-2, P=0.05). Perceived social support prior to surgery as given by the MOS score was significantly related to better scores for all QoL measures.
Analyses of missing data
Analyses of missing data were performed separately for patients who had two or more consecutive missing QoL scores up to and including the 12-month evaluation (due to loss to follow-up), and for patients with intermittent missing QoL scores. Ten patients were loss-to-follow-up up during the study period; two of them withdrew their consent, one relocated, and the remaining 7 missed follow-up appointments and could not be reached by telephone. No significant difference in the number of loss-to-follow-ups was found between SL and L patients [6 (60%) vs. 4 (40%), P=0.75] within this group.
For patients with intermittent missing data (Table 1), a longitudinal logistic regression model was used to compare missingness patterns between SL and L by creating a binary variable (rij=1 if the QoL observation was missing; rij=0 if it was obtained) as the dependent variable in a marginal model using generalized estimating equations. The predictor variables included categorical time for the missing observation (with T0 as the reference), extent of surgery (SL or L), and the interactions of categorical time and extent of surgery. The global chi-squared (χ2) test showed no significant differences in the likelihood of missing data between the SL and L patients, nor in the interaction of time and surgical groups [Surgery extent: =1.62, P=0.20; Surgery extent x time: =5.05, P=0.17]. The results of these analyses supported that patterns for loss-to-follow-up and intermittent missing data were similar between surgical groups. Therefore, in this study the missing data patterns themselves may not lead to serious biases in the estimation of surgical differences in QoL over time (27).
Discussion
Physical health (PCS) scores decreased within the first two postoperative months for both SL and L patients, and significantly more for L patients. Over the remaining 10 months, both SL and L patients rebounded significantly. While no significant changes were detected in lung cancer symptom (FACT-LCS) scores during the first two months for either group, SL patients reported significantly better scores a year after surgery compared to baseline while L patients did not. Mental health summary (MCS) and depression (PHQ-2) scores remained stable throughout the postoperative 12-month period. GAD-2 anxiety scores decreased significantly in the first two postoperative months in both groups, and remained stable thereafter. The 12-month scores for PCS and GAD-2 were significantly better than the baseline scores for both SL and L patients.
This study is the first to examine changes in multiple validated measures of QoL among early stage NSCLC surgical patients in a longitudinal prospective design which allowed for identification of critical postoperative changes in patient-specific trajectories during the first postoperative year. It revealed that interventions to improve physical QoL particularly during the first two postsurgical months might be most important as the lowest PCS score was observed during that time period for both SL and L patients. The physical score findings are consistent with those of our previous study (9) which also showed a decrease in physical health after surgery. The current study, however, allowed us to pinpoint a time period when intervention might be particularly beneficial—the first two postoperative months.
We confirmed that L patients fared worse in terms of PCS as compared with SL patients, a difference previously identified (9). Cancer-related symptoms, as measured by FACT-LCS, worsened slightly for L patients while improving slightly for SL patients; although these differences were not statistically significant. These QoL findings point to particular needs among L patients regarding pain management and physical functioning early in their recovery. Furthermore, the short-term negative impact on QoL of L patients should also be recognized when making surgical treatment decisions and in post-surgical management.
Unlike the other QoL measures, anxiety appeared to significantly improve within the first two postoperative months, and then stabilized over time. We speculate that the removal of the malignancy caused relief as compared with pre-surgical worries regarding morbidity and mortality related to the surgery and its efficacy. Other aspects of mental health, such as stigma and isolation were not assessed within the context of the current study, but have appeared as important considerations in qualitative analyses (7), and should be considered in the future. Also, it is possible that certain subgroups of early stage lung cancer surgical patients, such as those with pre-existing mental health diagnoses, exhibit lower QoL after surgery. Such patients might benefit from stronger postsurgical mental health intervention, as postsurgical QoL has been found to be lower among early stage patients who have higher pre-surgical depression and anxiety scores (14,16).
We also found significant differences between women and men for lung cancer symptoms (FACT-LCS) and anxiety (GAD-2) scores. Such sex-related differences were also identified in the focus groups (7) and need to be considered in postsurgical care. Although social support did not differ between SL and L patients at baseline, more perceived social support was a significant factor for all QoL measures used in this report. The literature indicates that social support as well as psychosocial and behavioral supportive interventions can have positive impact on post-surgical QoL (31,32). Thus, future research should consider whether social support could potentially be an effect modifier of the impact of extent of surgery so that the impact on QoL of more invasive surgery could be attenuated by increased social support.
A limitation of this report is missing data at baseline, which raises concerns about the validity of the average QoL differences and degree of relative change. Postoperative recruitment of patients accounted for 32% of the missing baseline QoL data, but these percentages did not significantly differ between SL and L patients (P=0.34). As patients had just received a diagnosis of lung cancer, reasons for failure to collect baseline data included patients having another appointment, needing to complete critical actions given this life-threatening diagnosis, personal hesitation, and nervousness. Many, however, agreed to participate after their treatment. Systematic differences by extent of surgery received would not be anticipated, given these considerations. Adjustment for multiple comparisons was not performed as this is our first exploratory study to compare the impact of SL and L on QoL using the IELCART database, this could lead to inflation of the Type I error rate. A subsequent study with preplanned hypotheses will be conducted to confirm our observed association as data accrued. Additional limitations include the possibility of residual confounding.
In conclusion, the current analyses support the idea of a critical postoperative window of two months where interventions could potentially improve physical health further. Treatments such as mind-body interventions that target pain and general mental health (e.g., relaxation, biofeedback) could be offered to patients, particularly lobectomy patients, who are experiencing symptoms. Such interventions have found to be effective in managing other chronic illnesses (33-36) and could be readily applied to early stage lung cancer surgical patients in an effort to improve overall QoL among this growing patient population.
Acknowledgments
Funding: This effort was made possible by generous grants from the Simons Foundation and philanthropic gifts from Sonia Gardner in loving memory of her father, Moise Lasry, and from Arthur and Selma Rabin.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-402
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-402). DFY reports other from Accumetra, other from GRAIL, outside the submitted work; In addition, DFY is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are non-exclusively licensed to General Electric. As an inventor of these patents, he is entitled to a share of any compensation which CRF may receive from its commercialization of these patents. CIH is a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since 2009, CIH does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF. CIH is the President and serve on the board of the Early Diagnosis and Treatment Research Foundation. CIH receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. Recipients include, I-ELCAP, among others. The funding comes from a variety of sources including philanthropic donations, grants and contracts with agencies (federal and non-federal), imaging and pharmaceutical companies relating to image processing assessments. The various sources of funding exclude any funding from tobacco companies or tobacco-related sources. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This prospective HIPAA-compliant study was conducted in accordance to the Declaration of Helsinki (as revised in 2013) and was approved by the Mount Sinai IRB (IRB approval #IF 2365016). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- CALGB 140503: A phase III randomized trial of lobectomy versus sublobar resection for small (≤2cm) peripheral non-small cell lung cancer (NCT00499330). Available online: http://www.cancer.gov/about-cancer/treatment/clinical-trials/search/view?cdrid=555324. Accessed July 17, 2019.
- M.D. Anderson Cancer Center International Randomized Study to Compare CyberKnife® Stereotactic Radiotherapy With Surgical Resection in Stage I Non-small Cell Lung Cancer. NCTT00840749, 2009. Available online: https://clinicaltrials.gov/ct2/show/NCT00840749. Accessed July 17, 2019.
- The Netherlands Organisation for Health Research and Development. A Randomized Clinical Trial of Surgery Versus Radiosurgery (Stereotactic Radiotherapy) in Patients With Stage IA NSCLC Who Are Fit to Undergo Primary Resection. NCT00687986, 2008. ; Accessed July 17, 2019.https://clinicaltrials.gov/ct2/show/NCT00687986
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- VA Office of Research and Development, Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR), In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [2017 Feb 6]. Identifier: NCT02984761. Accessed July 17, 2019.http://clinicaltrials.gov/show/NCT02984761NLM
- Schwartz RM, Gorbenko K, Kerath SM, et al. Thoracic surgeon and patient focus groups on decision-making in early-stage lung cancer surgery. Future Oncol 2018;14:151-63. [Crossref] [PubMed]
- Yip R, Taioli E, Schwartz R, et al. A Review of Quality of Life Measures used in Surgical Outcomes for Stage I Lung Cancers. Cancer Invest 2018;36:296-308. [Crossref] [PubMed]
- Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14:37-44. [Crossref] [PubMed]
- Schwartz RM, Yip R, Flores RM, et al. The impact of resection method and patient factors on quality of life among stage IA non‐small cell lung cancer surgical patients. J Surg Oncol 2017;115:173-80. [Crossref] [PubMed]
- Schwartz RM, Alpert N, Rosenzweig K, et al. Changes in quality of life after surgery or radiotherapy in early-stage lung cancer. J Thorac Dis 2019;11:154. [Crossref] [PubMed]
- Wolff HB, Alberts L, Kastelijn EA, et al. Differences in longitudinal health utility between stereotactic body radiation therapy and surgery in stage I non–small cell lung Cancer. J Thorac Oncol 2018;13:689-98. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Ostroff JS, Krebs P, Coups EJ, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer 2011;71:103-8. [Crossref] [PubMed]
- Koczywas M, Williams AC, Cristea M, et al. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol 2013;20:1788-97. [Crossref] [PubMed]
- Park S, Kang CH, Hwang Y, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer. Eur J Cardiothorac Surg 2016;49:e16-21. [Crossref] [PubMed]
- Flores R, Taioli E, Yankelevitz DF, et al. Initiative for early lung cancer research on treatment: development of study design and pilot implementation. J Thorac Oncol 2018;13:946-57. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193-203.
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705-14. [Crossref] [PubMed]
- Henschke CI, Yip R, Smith JP, et al. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 2016;207:1176-84. [Crossref] [PubMed]
- Ware Jr JE, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Lincoln, RI: QualityMetric, Inc., 2000.
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy—Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy–Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 2002;55:285-95. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB, et al. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics 2009;50:613-21. [PubMed]
- Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317-25. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. [Crossref] [PubMed]
- Yang X, Shoptaw S. Assessing missing data assumptions in longitudinal studies: an example using a smoking cessation trial. Drug Alcohol Depend 2005;77:213-25. [Crossref] [PubMed]
- Naumova EN, Must A, Laird NM. Tutorial in biostatistics: evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol 2001;30:1332-41. [Crossref] [PubMed]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons, 2012.
- Gałecki A., Burzykowski T. Linear mixed-effects model. In: Linear Mixed-Effects Models Using R. Springer, New York, NY: Springers Texts in Statistics, 2013.
- Huang FF, Yang Q, Zhang J, et al. The structural equation model on self-efficacy during post-op rehabilitation among non-small cell lung cancer patients. PLoS One 2018;13:e0204213. [Crossref] [PubMed]
- Raz DJ, Sun V, Kim JY, et al. Long-term effect of an interdisciplinary supportive care intervention for lung cancer survivors after surgical procedures. Ann Thorac Surg 2016;101:495-502. [Crossref] [PubMed]
- Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 2010;78:169-83. [Crossref] [PubMed]
- Benson H, Proctor W. Relaxation revolution: The science and genetics of mind body healing. New York, NY: Simon and Schuster, 2010.
- Vranceanu AM, Gonzalez A, Niles H, et al. Exploring the effectiveness of a modified comprehensive mind-body intervention for medical and psychologic symptom relief. Psychosomatics 2014;55:386-91. [Crossref] [PubMed]
- Vranceanu AM, Riklin E, Merker VL, et al. Mind-body therapy via videoconferencing in patients with neurofibromatosis: An RCT. Neurology 2016;87:806-14. [Crossref] [PubMed]


