Risk factors for venous thromboembolism and evaluation of the modified Caprini score in patients undergoing lung resection
Introduction
Surgical resection is a very important therapeutic modality for some lung diseases (1) but is associated with an increased risk of venous thromboembolism (VTE) after surgery (2) and increase complications and cost (3). Several results indicated that thoracic surgery patients are in one of the highest risk groups for VTE (4) and the incidence of VTE after lung resection ranges from 5% to 15.2% (5,6). Our previous study has shown that the incidence of VTE in patients undergoing lung resection without prophylaxis is about 11.5% (7). In addition, postoperative VTE leads to an 8-fold increase in mortality rates after lung cancer resection (8).
Risk factors for VTE include cancer-related factors, treatment-related factors, patient-related factors and biomarkers (9). According previous studies, major risk factors for VTE include cancer type, intravenous tumor invasion, chemotherapy, surgery, intravenous catheterization, advanced age, obesity, long-term bedridden status and trauma (10,11). Previous studies have shown that certain laboratory parameters are associated with an elevated risk of VTE, including white blood cell (WBC) counts (12) and high platelet (PLT)counts (13,14). The coagulation state of patients undergoing lung resection will be changed due to complications, tumors, and surgery (15). And the hypercoagulable state increases after operation, persists for at least 1 month, and returns to the baseline level after 6–12 months (16,17). Coagulation biomarkers can reflect coagulation activation and fibrinolysis, such as high levels of D-dimer are independent predictors of VTE in cancer patients (18). In the Khorana VTE risk score (KRS) model, the risk of developing VTE increased in patients with D-dimer values of ≥1.44 µg/mL (19).
Caprini score is widely used in many surgical specialties to identify high-risk patients with VTE (20). In recent years, the modified Caprini score has been used to assess the risk of VTE after resection of lung cancer (2). The study showed that the higher the score, the higher the incidence of VTE: low risk (score 0–4, 0%), moderate risk (score 5–8, 1.7%) and high risk (score ≥9, 10.3%) (2).
Currently, an experts consensus for the evaluation and prevention of VTE after lung cancer resection has been published (21). However, there are also some limitations need to be considered. Most of the recommendations are based on general surgery and other subprofessional literature, rather than thoracic surgery (22). The purpose of this study was to identify the risk factors of patients after lung surgery and evaluate the modified Caprini score, so as to provide reference for screening high-risk groups of VTE. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1279).
Methods
Subjects and study design
In this retrospective study, newly diagnosed lung disease patients hospitalized between July 2016 and December 2017 were selected at the Beijing Chao-Yang Hospital. Malignant tumors and benign diseases were included, among which benign diseases mainly included presumed malignant nodules, pulmonary vesicles, bronchiectasis and so on. Patients will be excluded from the study if they have one of following conditions: current VTE, perioperative prophylactic anticoagulation and insufficient clinical data. The application of perioperative prophylactic anticoagulation was not standard, which make it difficult to accurately evaluate its effect on VTE, so these patients were not included in this study. All patients received physical prophylaxis, including ankle pump exercise, graduated compress stocking (GCS), also went to the ground as early as possible after operation. This study was approved by the Beijing Chao-Yang Hospital Institutional Review Board. All the patients signed the informed consent preoperatively. The study complies with the Helsinki declaration (as revised in 2013). The follow-up period ended when the patient was discharged from the hospital.
We used electronic medical records to collect the following clinical information: age, sex, weight, height, body mass index (BMI), comorbidities (hypertension, diabetes mellitus, cardiovascular disease, and chronic pulmonary disease), smoking history, surgical information (surgical procedure, surgical approach, duration of operation), tumor pathology, metastasis, tumor staging, site of thrombosis, date of thromboembolism diagnosis, laboratory data [WBC count, PLT count, lymphocyte count, mean platelet volume (MPV), low density lipoprotein (LDL), blood glucose, antithrombin (AT), fibrin degradation products (FDP), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FBG), thrombin time (TT) and D-dimer concentration], and concomitant drug use.
In this study, DVT events were confirmed by duplex ultrasonography, and PE events were confirmed by computed tomography pulmonary angiography (CTPA). All patients were screened for DVT by duplex ultrasonography of both lower extremities once before and after operation. If the patient has typical PE symptoms (chest pain, haemoptysis, dyspnoea or persistent hypoxaemia), high Caprini score (≥9) or newly diagnosed DVT after operation, CTPA would be performed.
Risk assessment for VTE
In the modified Caprini score, the risk of VTE was evaluated on the following clinical parameters (2): age [40–59 (y), 1 points; 60–74 (y), 2 points; ≥75 (y), 3 points], abnormal pulmonary function, acute myocardial infarction (<1 mo), BMI ≥30 (kg/m2), congestive heart failure (<1 mo), history of inflammatory bowel disease, history of prior major surgery (<1 mo), complications of pregnancy, oral contraceptive use or HRT, sepsis (<1 mo), serious acute lung disease (<1 mo), swollen legs (current), varicose veins, central venous access, confined to bed (>72 h), major open surgery (≥45 min), present cancer, prior cancer (except nonmelanoma skin), history of VTE, family history of VTE, chemotherapy, positive anticardiolipin antibody, positive Lupus anticoagulant, acute spinal cord injury (<1 mo), major surgery ≥6 h. All variables included in the modified Caprini score were collected. In order to assess the applicability of the risk assessment, we classified patients into two groups according to VTE event: VTE group and non-VTE group.
We selected the following factors for risk analysis: age, sex, weight, BMI, comorbidities (hypertension, diabetes mellitus, cardiovascular disease, chronic pulmonary disease), smoking history, surgical procedure, surgical approach, duration of operation, tumor pathology, cancer metastasis, tumor stage and laboratory data (WBC count, PLT count, lymphocyte count, MPV, LDL, blood glucose, AT, FDP, PT, APTT, FBG, TT and D-dimer concentration). The VTE risk of patients varies dynamically with treatment, so we collected the laboratory data before operation, the first day, the third day and the fifth day after operation. At the same time, surgery has a great impact on these factors, so we included the data from the first day after operation in the analysis.
Statistical analysis
Data are presented as means ± standard deviation (SD) or medians with ranges (minimum value-maximum value) where appropriate. T test, Mann-Whitney U test or Fisher’s exact test were used to analyze the difference between the two groups. Any variable with a P value of 0.2 in the univariate analysis was included in multivariate analysis. Multivariate logistic regression analysis was used to identify the risk factors related to VTE. We used the area under the receiver operating characteristic (ROC) curve to discriminate patients between the patients with VTE and those without VTE. P value <0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics version 21.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics and VTE prevalence
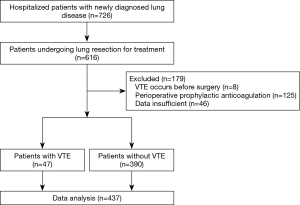
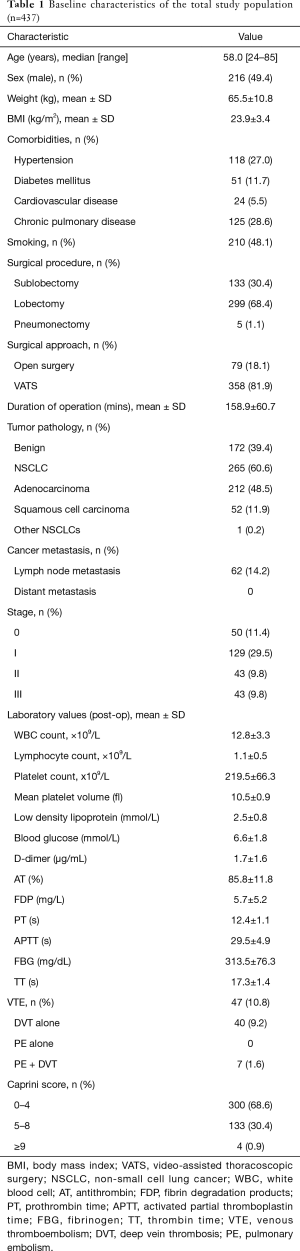
A total of 726 patients with newly diagnosed lung diseases were admitted to our hospital. Of these patients, 616 were hospitalized for lung resection. Eight patients were excluded because of VTE before surgery, 125 patients were excluded because they received prophylactic anticoagulant therapy, 46 patients were excluded due to insufficient data. Finally, 437 eligible patients were enrolled in this study (Figure 1). The baseline characteristics of patients are shown in Table 1. The median age of patients is 58.0 years, and 49.4% (n=216) of them were men. The surgical procedures included sublobectomy in 133 patients (30.4%), lobectomy in 299 (68.4%) and pneumonectomy in 5 (1.1%). In sublobectomy, 47 cases were segmental lobectomy and 86 cases were wedge resection. The surgical approach includes video-assisted thoracoscopic surgery (VATS) in 358 patients (81.9%) and open surgery in 79 (18.1%). Pathologic results shown that benign lesions account for 39.4% (n=172) and malignant tumors account for 60.6% (n=265). Malignancies included 48.5% (n=212) adenocarcinoma, 11.9% (n=52) squamous cell carcinoma and 0.2% (n=1) other non-small cell lung cancers (NSCLC). Among them, 14.2% (n=62) had lymph node metastasis. As for tumor staging, stage 0, stage I, stage II and stage III accounted for 11.4% (n=50), 29.5% (n=129), 9.8% (n=43) and 9.8% (n=43), respectively.
Full table
Overall, VTE events occurred in 47 of the 437 patients (10.8%). Forty patients (9.2%) developed DVT alone, 7 (1.6%) developed both DVT and PE, and no case developed PE alone. According to the modified Caprini risk assessment model (RAM), there were 300 patients at low risk (0–4 points), 133 patients at moderate risk (5–8 points), and 4 patients at high risk (≥9 points). The corresponding VTE incidence was 12.3% (37/300), 7.5% (10/133) and 0% (0/4), respectively (P>0.05).
Risk factors associated with VTE
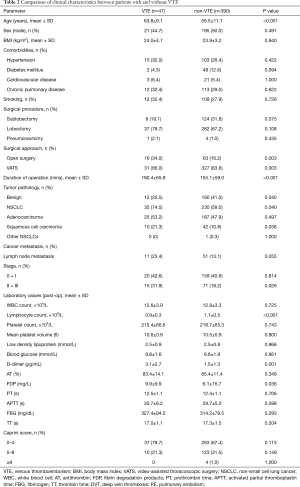
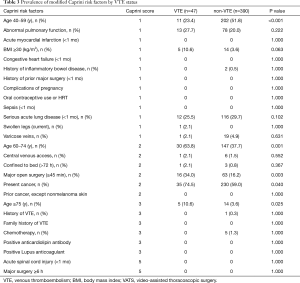
Table 2 shows the characteristics of patients with or without VTE. Univariate analysis showed significant differences between two groups in the following parameters: age (P<0.001), surgical approach (P=0.003), duration of operation (P<0.001), NSCLC (P=0.040), squamous cell carcinoma (P=0.036), tumor staging II + III (P=0.026), lymphocyte count (P<0.001), D-dimer concentration (P=0.003), and FDP (P=0.011). There were also significant differences among age, major open surgery (≥45 min) and present cancer in the modified Caprini RAM (Table 3). Considering the multicollinearity, NSCLC, squamous cell carcinoma and FDP were excluded. Surgical approach and the duration of operation were separately included in multivariate analysis.
Full table
Full table
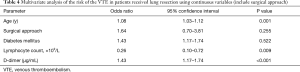
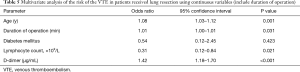
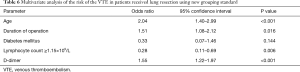
Firstly, we included the surgical approach into the multivariate analysis, and the results showed that it was not an independent risk factor for VTE (Table 4). After that, we included the duration of operation into multivariate analysis to identify the independent risk factors for VTE (Table 5). As a result, age [odds ratio (OR), 1.08; 95% confidence interval (CI), 1.03–1.12; P=0.001], duration of operation (OR, 1.01; 95% CI, 1.00–1.01, P=0.031), lymphocyte count (OR, 0.31; 95% CI, 0.12–0.84, P=0.021) and D-dimer concentration (OR, 1.42; 95% CI, 1.18–1.70, P<0.001) were significantly associated with VTE. The cut-off values for lymphocyte count and D-dimer concentration employing ROC analysis were 1.15×109 (/L) [area under the curve (AUC), 0.64] and 1.37 µg/mL (AUC, 0.73), respectively. The AUC of the modified Caprini score is 0.474 (P=0.558). At last, we grouped the age, duration of operation, lymphocyte count and D-dimer concentration for multivariate logistic analysis. Every 10 years of increase in age was divided into one layer, each hour of operation time was divided into one layer, and D-dimer was divided into one layer with each increase of 1.44 µg/mL (19). There is no report on the cut-off value of lymphocyte count in lung resection patients. Therefore, we used this result to group in multivariate logistic analysis. As a result, age (OR, 2.04; 95% CI, 1.40–2.99), duration of operation (OR, 1.51; 95% CI, 1.08–2.12), lymphocyte count (OR, 0.28; 95% CI, 0.11–0.69), and D-dimer concentration (OR, 1.55; 95% CI, 1.22–1.97) were significantly associated with VTE (Table 6).
Full table
Full table
Full table
In order to further evaluate the effectiveness of the modified Caprini score, we conducted a subgroup analysis (Table 7). Among all patients, the incidence of VTE in low-risk group (0–4), moderate-risk group (5–8) and high-risk group (≥9) was 12.3% (37/300), 7.5% (10/133) and 0% (0/4), respectively. In addition, similar results were obtained in NSCLC and benign disease groups.

Full table
Discussion
In this study, the total incidence of postoperative VTE was 10.8%, of which DVT, PE and DVT + PE accounted for 85.1%, 0% and 14.9%, respectively. We identified four independent factors associated with VTE: age, duration of operation, lymphocyte count, D-dimer. Among them, lymphocyte count is the protective factor of VTE, and the others are the risk factors. The cut-off values of lymphocyte count and D-dimer employing ROC analysis were 1.15×109/L (AUC, 0.64), 1.37 µg/mL (AUC, 0.73), respectively.
Advanced age is known to be an independent risk factor for VTE (23-25). In this study, we obtained similar results: age (OR, 2.04; 95% CI, 1.40–2.99) was significantly associated with VTE in lung resection patients. Because every 10 years of age is divided into one layer, the result means that for every 10 years of age, the incidence of VTE increases by 1.04 times.
Duration of operation was independently associated with an increased likelihood of VTE development (26-29). The explanation for linking operation time to VTE is multifactorial. Immobility during long surgical procedures can result in the simultaneous presence of blood stasis, increased coagulation, and endothelial damage (30). In addition, prolonged surgery leads to inflammation and oxidative stress, which independently contributing to thrombosis formation (31). In this study, duration of operation was associated with VTE with an OR of 1.51/hour. In craniotomies patients, surgical duration longer than 4 h has been identified as a risk factor of VTE (27). Tran et al. reported an OR of 4.36 for operation time >3 h in patients undergoing mastectomy without reconstruction (32).
Lymphocyte count (OR, 0.28; 95% CI, 0.11–0.69, P=0.006) was a protective factor for VTE in lung resection patients in this study. The cut-off values for lymphocyte count was 1.15×109 (/L). In recent years, more and more attention is has been paid to the role of inflammatory markers in VTE (33,34). Inflammation may interfere with several stages of hemostasis by activating coagulation or by inhibiting fibrinolysis and anticoagulation pathways (35). Lymphopenia is the result of margination and redistribution of lymphocytes in the lymphatic system, accompanied by accelerated apoptosis (36). In this study, we also analyzed WBC count, Hb, PLT count, MPV and other biomarkers, but there was no statistical difference. These biomarkers can not only explain the pathogenesis of thrombosis, but also serve as useful diagnostic markers.
High D-dimer levels are recognized as biomarker associated with the risk of developing VTE (37-39). D-dimer is the degradation product of cross-linked fibrin, indicating the global coagulation activation and fibrinolysis. Elevated levels of D-dimer most probably reflect the hypercoagulable state, which could be affected by anticoagulation (40). In this study, we tried to evaluate the risk of postoperative VTE, and the change of D-dimer was clearly correlated with surgery. So we analyzed the level of D-dimer on the first day after operation, and the results showed that it was an independent risk factor for postoperative VTE. When D-dimer concentration increased by 1.44 µg/mL, the risk of VTE increased by 0.55 times. The cut-off values for D-dimer concentration was 1.37 µg/mL (AUC =0.732, P<0.001), and its sensitivity and specificity are 0.780 and 0.591 respectively. The result is similar to that in the KRS (19).
At present, modified Caprini score (2) and Rogers score (41) are two commonly used risk assessment models in surgical patients. The Rogers score does not take duration of operation into account. In the modified Caprini score, there are two items about operation time: major open surgery (≥45 min) and major surgery ≥6 h. In this study, only 79 (8.9%) patients underwent open surgery, and the operation time of all patients was no more than 6 hours. Duration of operation, as a very important risk factor, had not been well evaluated. In this study, there were only 4 patients in high-risk group, and the incidence of VTE in low-risk group (0–4), moderate-risk group (5–8) and high-risk group (≥9) was 12.3% (37/300), 7.5% (10/133) and 0% (0/4), respectively (P>0.05). In the subgroup analysis, the results were similar. And the AUC of the modified Caprini score is 0.474 (P=0.558). These results suggest that the modified Caprini score is not effective enough for VTE risk stratification in patients after lung surgery. Hachey et al. retrospectively assigned modified Caprini score to 232 patients undergoing pneumonectomy and proved that modified Caprini score can stratify the risk of VTE (2). By comparing patients information, patients in our study were less likely to undergo open surgery (79/437 vs. 162/232) and central venous access (7/437 vs. 68/232), which may be the reason for the small number of high-risk group and the inefficiency of the modified Caprini score in our study.
In this retrospective study, the follow-up period ended when the patients were discharged from the hospital. The median time from ultrasound to operation is 3 days, and the median days of receiving ultrasound, CTPA and hospitalization after operation were 5, 7 and 7 days, respectively. The length of follow-up is closely related to the diagnosis of VTE. On the one hand, Moghadamyeghaneh et al. reported that the first week after operation was the most common time for postoperative VTE (42). On the other hand, Thomas et al. reported that about 40% of VTE occurred after discharge (43). Besides, the missed diagnosis of ultrasound examinations may also underestimate the incidence of VTE. Overall, the incidence of postoperative VTE in our study may be underestimated, which may also lead to deviations in the results of the analysis. Therefore, this is only the result of our single-center study, and the results still need to be verified by a large sample of multicenter studies.
This study has several limitations. First, this study is a single-center, retrospective study, which may cause bias. Second, we did not perform CTPA in all patients to avoid side effects and reduce costs, which might cause small PEs not to be identified. Finally, we did not include patients who received prophylactic anticoagulation in this study, which may also cause some bias.
In conclusion, our study identified four significant factors for VTE in patients undergoing lung resection. The results suggested that the modified Caprini score may not accurately assess the risk of VTE after pulmonary surgery. Risk assessment models based on clinical characteristics and biomarkers will better identify the high-risk groups of VTE who can really benefit from prophylactic anticoagulation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1279
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1279
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1279
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1279). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Research Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (ID: 2017-Ke-1). All the patients signed the informed consent preoperatively. The study complies with the Helsinki declaration (as revised in 2013). The study outcomes will not affect the future management of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003;123:2096-103. [Crossref] [PubMed]
- Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg 2016;151:37-44.e1. [Crossref] [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-e496S.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446-55. [Crossref] [PubMed]
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975-80. [Crossref] [PubMed]
- Shah DR, Wang H, Bold RJ, et al. Nomograms to predict risk of in-hospital and post-discharge venous thromboembolism after abdominal and thoracic surgery: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Surg Res 2013;183:462-71. [Crossref] [PubMed]
- Song C, Shargall Y, Li H, et al. Prevalence of venous thromboembolism after lung surgery in China: a single-centre, prospective cohort study involving patients undergoing lung resections without perioperative venous thromboembolism prophylaxis†. Eur J Cardiothorac Surg 2019;55:455-60. [Crossref] [PubMed]
- Trinh VQ, Karakiewicz PI, Sammon J, et al. Venous thromboembolism after major cancer surgery: temporal trends and patterns of care. JAMA Surg 2014;149:43-9. [Crossref] [PubMed]
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839-47. [Crossref] [PubMed]
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-77S.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med 2012;9:e1001275. [Crossref] [PubMed]
- Duman H, Özyurt S, Erdoğan T, et al. The role of serum bilirubin levels in determining venous thromboembolism. J Vasc Surg Venous Lymphat Disord 2019;7:635-9. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822-9. [Crossref] [PubMed]
- Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost 2010;8:114-20. [Crossref] [PubMed]
- Mulder MB, Proctor KG, Valle EJ, et al. Hypercoagulability After Resection of Thoracic Malignancy: A Prospective Evaluation. World J Surg 2019;43:3232-8. [Crossref] [PubMed]
- Thorson CM, Van Haren RM, Ryan ML, et al. Persistence of hypercoagulable state after resection of intra-abdominal malignancies. J Am Coll Surg 2013;216:580-9; discussion 589-90. [Crossref] [PubMed]
- Van Haren RM, Valle EJ, Thorson CM, et al. Long-term coagulation changes after resection of thoracoabdominal malignancies. J Am Coll Surg 2014;218:846-54. [Crossref] [PubMed]
- Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013;122:2011-8. [Crossref] [PubMed]
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902-7. [Crossref] [PubMed]
- Qaseem A, Chou R, Humphrey LL, et al. Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011;155:625-32. [Crossref] [PubMed]
- Li H, Jiang G, Bölükbas S, et al. The Society for Translational Medicine: the assessment and prevention of venous thromboembolism after lung cancer surgery. J Thorac Dis 2018;10:3039-53. [Crossref] [PubMed]
- Van Haren RM, Litle VR. Venous thromboembolism events after thoracic surgery: global steps toward prevention. J Thorac Dis 2018;10:S3058-9. [Crossref] [PubMed]
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 2017;117:219-30. [Crossref] [PubMed]
- Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer 2013;49:1404-13. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 2007;25:70-6. [Crossref] [PubMed]
- Kim JY, Khavanin N, Rambachan A, et al. Surgical duration and risk of venous thromboembolism. JAMA Surg 2015;150:110-7. [Crossref] [PubMed]
- Bekelis K, Labropoulos N, Coy S. Risk of Venous Thromboembolism and Operative Duration in Patients Undergoing Neurosurgical Procedures. Neurosurgery 2017;80:787-92. [Crossref] [PubMed]
- Qiu CS, Jordan SW, Dorfman RG, et al. Surgical Duration Impacts Venous Thromboembolism Risk in Microsurgical Breast Reconstruction. J Reconstr Microsurg 2018;34:47-58. [Crossref] [PubMed]
- Chan MM, Hamza N, Ammori BJ. Duration of surgery independently influences risk of venous thromboembolism after laparoscopic bariatric surgery. Surg Obes Relat Dis 2013;9:88-93. [Crossref] [PubMed]
- Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ 2002;325:887-90. [Crossref] [PubMed]
- Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387-91. [Crossref] [PubMed]
- Tran BH, Nguyen TJ, Hwang BH, et al. Risk factors associated with venous thromboembolism in 49,028 mastectomy patients. Breast 2013;22:444-8. [Crossref] [PubMed]
- Reiter M, Bucek RA, Koca N, et al. Deep vein thrombosis and systemic inflammatory response: a pilot trial. Wien Klin Wochenschr 2003;115:111-4. [Crossref] [PubMed]
- Matos MF, Lourenço DM, Orikaza CM, et al. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6-174GC, IL-8-251AT and MCP-1-2518AG in the risk of venous thromboembolism: a case-control study. Thromb Res 2011;128:216-20. [Crossref] [PubMed]
- Bakirci EM, Topcu S, Kalkan K, et al. The role of the nonspecific inflammatory markers in determining the anatomic extent of venous thromboembolism. Clin Appl Thromb Hemost 2015;21:181-5. [Crossref] [PubMed]
- Farah R, Nseir W, Kagansky D, et al. The role of neutrophil-lymphocyte ratio, and mean platelet volume in detecting patients with acute venous thromboembolism. J Clin Lab Anal 2020;34:e23010. [Crossref] [PubMed]
- Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2009;27:4124-9. [Crossref] [PubMed]
- Tian B, Song C, Li H, et al. The significance of perioperative coagulation and fibrinolysis related parameters after lung surgery for predicting venous thromboembolism: a prospective, single center study. J Thorac Dis 2018;10:2223-30. [Crossref] [PubMed]
- Streiff MB, Agnelli G, Connors JM, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis 2016;41:32-67. [Crossref] [PubMed]
- Weitz IC, Israel VK, Waisman JR, et al. Chemotherapy-induced activation of hemostasis: effect of a low molecular weight heparin (dalteparin sodium) on plasma markers of hemostatic activation. Thromb Haemost 2002;88:213-20. [Crossref] [PubMed]
- Rogers SO Jr, Kilaru RK, Hosokawa P, et al. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1211-21. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Carmichael JC, et al. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg 2014;18:2169-77. [Crossref] [PubMed]
- Thomas DC, Arnold BN, Hoag JR, et al. Timing and Risk Factors Associated With Venous Thromboembolism After Lung Cancer Resection. Ann Thorac Surg 2018;105:1469-75. [Crossref] [PubMed]