
Impact of the interferon-γ release assay and glomerular filtration rate on the estimation of active tuberculosis risk before bronchoscopic examinations: a retrospective pilot study
Introduction
Bronchoscopic examinations are essential for diagnosing many thoracic diseases. Various gene alterations have been reported in lung cancer, and gene mutations and fusion genes can be detected by collecting lung tissue samples via bronchoscopy (1). However, as coughing is triggered during and after bronchoscopy, it is imperative to take measures against nosocomial infections, especially for airborne infections like tuberculosis (TB), measles, and chickenpox. TB remains a leading disease worldwide, with approximately 10 million cases reported annually (2). It is easy to diagnose patients with TB by examining the sputum before bronchoscopy when they present with typical symptoms and imaging findings. However, there are other disorders presenting with similar clinical and imaging findings, including lung abscesses, mycoses, nontuberculous Mycobacteria, and malignancies, although they can occur concomitantly in some cases (3). Thus, bronchoscopy is needed to differentiate these diseases, especially among patients who have scanty or no sputum production. Notably, bronchoscopy has been reported to correlate with a 20-fold elevated risk of TB dissemination before and after the procedure (4). Because lung TB is a stigmatized disease, it is important to prevent TB dissemination during bronchoscopic examination. Bronchoscopy is ideally performed while using personal protective equipment, including an N95 mask, designed to achieve a very close facial fit and very efficient filtration of airborne particles. However, N95 masks are in finite supply. Thus, these high-efficiency masks should be used only for potential cases of airborne infection on bronchoscopy and the ability to stratify patients before bronchoscopy is important for making this decision.
Infection with Mycobacterium tuberculosis (MTB) has two different states: active and latent. To evaluate the infection status of TB, the Interferon (IFN)-γ release assay (IGRA) has recently replaced the traditional tuberculin skin test (5). A positive IGRA result implies an existing TB infection, but does not distinguish the two states (6). Latent TB infection (LTBI) is defined as a positive IGRA result with no evidence of MTB in the smear and culture, regardless of parenchymal abnormalities (7). Recent research has elucidated the diversity of TB by demonstrating heterogeneity in the host, granuloma composition, and causative bacteria (8). LTBI can vary extensively from an entirely inactive state to a nearly active state. Some studies have identified that recent immigrants from high-prevalence countries, drug users, nursing home residents, and homeless people are at high risk of TB morbidity (9). Other studies have reported individual susceptibility factors for the increased probability of active disease from LTBI (10,11), including close contact with patients with TB, human immunodeficiency virus (HIV) infection, pulmonary parenchymal abnormalities, young or advanced age, smoking, weight loss, post gastrectomy, chronic renal failure, malignancies, poorly controlled diabetes, moderate dose of corticosteroids (≥15 mg of prednisone per day), and immunosuppressive therapy, including TNF-α inhibitors. Currently, the only way to predict active TB before bronchoscopy is an intensive checkup using a chest X-ray or computed tomography scan, but even this is not always accurate. Several studies indicate that certain combined risk factors increase the likelihood of active TB when a positive IGRA is returned (3,12). The present study is the first to investigate the efficacy of combining IGRA with clinical risk factors before bronchoscopy to estimate active TB.
Methods
Population and data collection
A cross-sectional study was conducted to investigate the efficacy of IGRA combined with clinical risk factors to estimate active TB before bronchoscopy at the Showa University Fujigaoka Hospital from January 2016 to March 2017. Patients undergoing bronchoscopy were included in the study. Patients were excluded if they had been diagnosed with active TB before bronchoscopic examination. We identified 205 inpatients, all of whom were on-time bronchoscopy. All clinical data were collected from the patients’ medical records on the day of bronchoscopy. Tobacco consumption was represented as pack-years, which is calculated by multiplying the number of packs of tobacco smoked per day by the number of years the person has smoked. The presence of several malignant diseases is a well-established risk factor for developing active TB (13). Although hematological, head and neck, and lung malignancies correlate with a high rate of developing TB, breast, gynecological, gastroenterological, urological, and thyroid cancers are independent of the disease. The current study was limited to hematological, head and neck, and lung malignancies. The estimated glomerular filtration rate (eGFR) of each participant was calculated using the following formula: eGFR (mL/min/1.73 m2) = 194 × serum creatinine −1.094 × age −0.287 × 0.739 (if female) (14). This study was carried out in accordance with the guidelines of the Helsinki Declaration (as revised in 2013). This study protocol was approved by the Institutional Ethics Committee of Showa University (approval no. F2019C11). The requirement for obtaining informed consent from the patients was waived because of the retrospective nature of this study.
IGRA testing
A QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis Inc., Valencia, CA, USA) test was performed as per the manufacturer’s guidelines. A QFT-GIT test is composed of three tubes: negative control, mitogen (positive control), and TB antigens. The concentration of IFN-γ in each tube was measured by enzyme-linked immunosorbent assay. Test results were reported as positive (IFN-γ for TB antigens minus negative control ≥0.35 IU/mL) or negative. Cases where the mitogen minus negative control was <0.5 IU/mL or the negative control was >8 IU/mL were excluded as indeterminate. All QFT-GIT results were available before bronchoscopy.
Bronchoscopic procedure—derived clinical isolates
We collected samples for MTB culture by scraping the site of the primary lesion with a curette or a brush, followed by flushing with 5 mL of saline. These lavage fluids were cultured in Mycobacteria growth indicator tubes and 2% Ogawa solid medium. Active TB was defined by a positive MTB culture.
Statistical analysis
Fisher’s exact test was used for univariate analysis of the association between two categorical variables. We evaluated the adjusted effects of multiple variables on active TB using a logistic regression model and presented the findings as an odds ratio (OR) with a 95% confidence interval (CI). P<0.05 was considered statistically significant. Multiple variables were then converted into a decision tree to estimate active TB. All statistical analyses were performed using JMP 13.0 software (SAS Institute, Cary, NC, USA).
Results
Patients’ characteristics
We enrolled 205 inpatients, all of whom were Japanese with no close contact with patients with active TB within at least a decade. No HIV antibody test was performed. The study cohort included no immigrants, drug users, or homeless people. Of the 205 patients, we excluded 60 because no IGRA results were available. IGRA results were obtained from 145 (70.7%); of these, we excluded 9 because of indeterminate IGRA results. Ultimately, 136 patients (58 males, 78 females; age at diagnosis: 28–96 years old) were statistically analyzed (Figure 1). Table 1 summarizes the patients’ characteristics. Of the 136 patients, 22 (16.2%) had diabetes mellitus, which was controlled within 7% hemoglobin A1c (HbA1c) in all cases, and 8 were receiving corticosteroid (less than 15 mg/day of prednisolone) for various indications. None of the patients were on any other immunosuppressants. Of the 136 patients, 36 (26.5%) had renal failure (defined as eGFR <60 mL/min/1.73 m2) and 77 (56.6%) had malignancies including lymphoma, pharyngeal cancer, laryngeal cancer, non-small-cell lung carcinoma (NSCLC), small-cell lung carcinoma (SCLC), and metastatic lung cancer. A positive MTB culture (active TB) was found in 5 patients (3.7%), 2 of whom were diagnosed with NSCLC and SCLC; of these, 80% (4 out of 5) had positive IGRA results (Table 2).
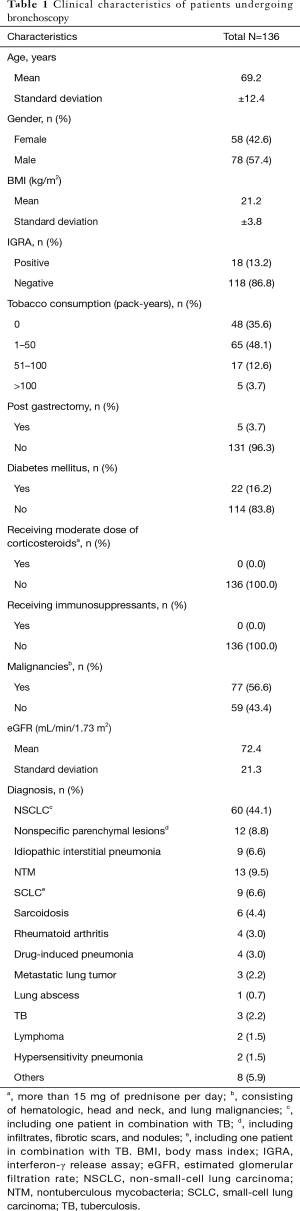
Full table

Full table
Multivariate analysis of the IGRA status and clinical features
In univariate analyses, the difference in active TB rates between positive IGRA (4 of 18; 22%) and negative IGRA (1 of 118; 0.8%) was especially evident (P=0.001). However, there was no significant association of active TB with age, gender, body mass index (BMI), smoking history, post gastrectomy, diabetes mellitus, malignancies, and renal function (Table S1). Table 3 presents the logistic regression models of factors relating to active TB on bronchoscopic examination. We used multivariate logistic regression models to control for the potential confounding effects of variables like age, gender, BMI, IGRA, tobacco consumption (pack-years), post gastrectomy, malignancies, and eGFR. TB prevalence correlated with IGRA result (OR for positive versus negative result: 72.7; 95% CI: 3.169–1668; P=0.007). When the IGRA result was combined with elevated eGFR per one-unit, the TB prevalence declined markedly (OR: 0.937; 95% CI: 0.882–0.996; P=0.038). Conversely, age (P=0.408), gender (P=0.613), BMI (P=0.804), tobacco consumption (P=0.825), post gastrectomy (P=0.512), and malignancies (P=0.733) were not significantly related to TB prevalence.
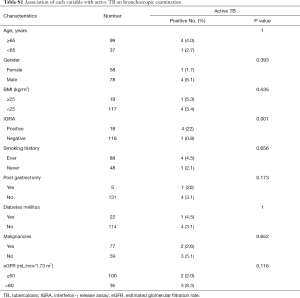
Full table

Full table
Decision tree for predicting active TB
We predicted active TB presence in patients undergoing bronchoscopy using a decision tree (Figure 2). Of the 136 patients, 18 had a positive IGRA result, and of these patients, 9 had eGFR <69.3 mL/min/1.73 m2 and were taken as probable TB cases. The value was determined automatically by the decision tree. Out of the 9 probable cases, 4 patients had active TB on culture results. There were no false negative predictions.
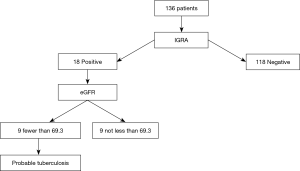
Discussion
Owing to the coughing stimulation during and after bronchoscopy, measures to control nosocomial infections are recommended. For the dissemination of TB, the incidence and duration of coughing may be more crucial than cumulative exposure time to patients with TB (5). In addition, the risk of dissemination during and after bronchoscopy could reach up to 20 times compared with no intervention (4). An IGRA result alone cannot determine which individuals will develop TB (6). In this study, we investigated the efficacy of combining IGRA with clinical findings to predict the presence of TB in patients undergoing bronchoscopy. Five patients were diagnosed with active TB, and multivariate analysis suggested that low eGFR and positive IGRA result markedly correlated with active TB. Moreover, a decision tree validated the use of these two variables to uncover probable active TB. Therefore, the results from two different statistical analyses were analogous. Our study suggests that as well as IGRA, renal function should be considered, especially for patients requiring bronchoscopy. Age, gender, BMI, tobacco consumption, post gastrectomy, and malignancies did not markedly correlate with active TB.
HIV infection is reportedly the most potent risk factor for developing active TB (10). However, the HIV infection rate in people aged 60 and above reached up to 5% in Japan (15), and according to a national survey, the HIV infection rate in patients with TB in 2017 was only 0.44% (2). Although we did not perform an HIV antibody test in this study, 80.9% (110 out of 136) of our patients were above 60 years of age. Hence, the HIV infection status was unlikely to exert a substantial impact on the estimation of TB presence in this study. Radiographic findings suggestive of a TB history are also a common risk factor for TB progression (10,11). Specifically, fibronodular changes correlate with a markedly higher risk of TB reactivation (16). Moreover, common abnormalities such as infiltrates, fibrotic scars, and nodules with or without calcification correlate with TB reactivation (17). As patients requiring bronchoscopy normally harbor some lung parenchymal involvement for investigation, we did not include radiological factors in this study. In some studies, poorly controlled diabetes has been proposed as a clinical risk factor for TB development (10,11), but in this study, no diabetic patient had an HbA1c >7%, and we had no obese diabetic patients (as defined by BMI >30 kg/m2). Hence, we did not include diabetes mellitus in the multivariate analysis in this study.
There is up to a 10% lifetime chance of TB reactivation (18), but the majority of individuals are clinically silent their entire lives. It is not yet clear why only a few patients develop active disease regardless of their immunological status. It has been reported in several studies that genetic factors affect TB outcome (19-21). Although environmental factors appear to contribute more than hereditary factors to TB progression, polygenic alterations may be related to the increased susceptibility or resistance to TB. In particular, the IFN signature correlates with active TB. Immunity dependent on IFN-γ, also called type II IFN, is crucial for protecting against TB, whereas type I IFN promotes TB infections by inhibiting IL-1β (22). In addition, the upregulation of type I IFN before TB infection correlates with poor infection outcome (23), suggesting inherent genetic differences in the immune response to infectious diseases. However, in a recent study, a protective role for type I IFN was suggested in active TB where no IFN- signaling was seen (24). Hence, the IFN interaction between type I and type II plays a pivotal role in TB infection. As IFN-γ levels are affected by renal dysfunction (25,26), a decline in eGFR could modify the individual susceptibility to TB infection in each immune status on the basis of genetic differences through a process of IFN transcription.
In this study, IGRA was positive in 18 patients, including 4 active TB and 14 LTBI patients. It should be noted that some clinical factors increase the probability of active disease developing from LTBI, and there is a subset of clinically defined LTBI, subclinical TB, which is a source of TB dissemination. The significance of preventive treatment of high-risk LTBI patients is highly publicized (10,11). Administration of isoniazid, rifampicin, or both is beneficial for preventing the progression to active disease. Thus, preventive anti-TB treatment may be desirable in a subset of patients requiring bronchoscopy. In contrast, transcriptional activation similar to that in active TB has been reported in up to 20% of patients with LTBI who can develop active disease (19). In fact, TB reactivation from LTBI can occur despite preventive therapy. Thus, a new preventive strategy is warranted. Although vaccination is considered an alternative therapy, the efficacy of Bacillus Calmette-Guérin, the only available vaccine, is limited only to infancy or school age, but not adulthood (27). New TB vaccines have recently been developed against LTBI in adolescents and adults (28,29), and vaccine therapy might replace preventive chemotherapy in LTBI in the future.
Conclusions
Bronchoscopy is extensively used for diagnosing pulmonary diseases. However, it is very important to prevent airborne infections during and after the procedure. This study investigates the prediction of active TB by using IGRA and eGFR results in patients undergoing bronchoscopy. The proposed strategy could identify patients who require antibiotic therapy for the prevention of TB or are in the active phase of TB. How renal dysfunction correlates with various transcriptional mechanisms in TB progression remains unclear. Hence, comprehensive clinical studies are warranted to extend the findings of this study.
Acknowledgments
We would like to thank Kunihiko Fukuchi for advice about bacteriological culture.
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-19-3653
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3653). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in accordance with the guidelines of the Helsinki Declaration (as revised in 2013). This study protocol was approved by the Institutional Ethics Committee of Showa University (approval no. F2019C11). The requirement for obtaining informed consent from the patients was waived because of the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [Crossref] [PubMed]
- Zumla A, George A, Sharma V, et al. The WHO 2014 Global tuberculosis report-further to go. Lancet Glob Health 2015;3:e10-2. [Crossref] [PubMed]
- Yamaguchi F, Minakata T, Miura S, et al. Heterogeneity of latent tuberculosis infection in a patient with lung cancer. J Infect Public Health 2020;13:151-3. [Crossref] [PubMed]
- Riley RL, Nardell EA. Clearing the air. The theory and application of ultraviolet air disinfection. Am Rev Respir Dis 1989;139:1286-94. [Crossref] [PubMed]
- Diel R, Loddenkemper R, Meywald-Walter K, et al. Comparative performance of tuberculin skin test, Quanti FERON-TB-Gold in tube assay, and T-SpotTB test in contact investigations for tuberculosis. Chest 2009;135:1010-8. [Crossref] [PubMed]
- Mack U, Migliori GB, Sester M, et al. LTBI: Latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956-73. [Crossref] [PubMed]
- Menzies D, Jahdali H, Otaibi B. Recent developments in treatment of latent tuberculosis infection Regimens for treatment of LTBI. Indian J Med Res 2011;133:257-66. [PubMed]
- Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol 2017;17:691-702. [Crossref] [PubMed]
- Dunlap NE, Bass J, Fujiwara P, et al. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:1376-95. [PubMed]
- Horsburgh CR, Rubin EJ. Latent tuberculosis infection in the United States. N Engl J Med 2011;364:1441-8. [Crossref] [PubMed]
- Diel R, Loddenkemper R, Zellweger JP, et al. Old ideas to innovate tuberculosis control: Preventive treatment to achieve elimination. Eur Respir J 2013;42:785-801. [Crossref] [PubMed]
- Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ 2020;368:m549. [Crossref] [PubMed]
- Cheng MP, Abou Chakra CN, Yansouni CP, et al. Risk of active tuberculosis in patients with cancer: a systematic review and meta-analysis. Clin Infect Dis 2017;64:635-44. [PubMed]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982-92. [Crossref] [PubMed]
- Yoshikura H. HIV/AIDS in Japan: route and age of infection that shaped the epidemics in 1987- 2016. Jpn J Infect Dis 2019;72:23-30. [Crossref] [PubMed]
- Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics 2017;37:52-72. [Crossref] [PubMed]
- Esmail H, Lai RP, Lesosky M, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2- 18 F.fluoro-D-glucose positron emission and computed tomography. Nat Med 2016;22:1090-3. [Crossref] [PubMed]
- Andrews JR, Noubary F, Walensky RP, et al. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis 2012;54:784-91. [Crossref] [PubMed]
- Berry MPR, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010;466:973-7. [Crossref] [PubMed]
- Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016;387:2312-22. [Crossref] [PubMed]
- Blankley S, Graham CM, Turner J, et al. The transcriptional signature of active tuberculosis reflects symptom status in extra-pulmonary and pulmonary tuberculosis. PLoS One 2016;11:e0162220. [Crossref] [PubMed]
- Novikov A, Cardone M, Thompson R, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J Immunol 2011;187:2540-7. [Crossref] [PubMed]
- Gideon HP, Skinner JA, Baldwin N, et al. Early whole blood transcriptional signatures are associated with severity of lung inflammation in Cynomolgus Macaques with Mycobacterium tuberculosis infection. J Immunol 2016;197:4817-28. [Crossref] [PubMed]
- Moreira-Teixeira L, Sousa J, McNab FW, et al. Type I IFN inhibits alternative macrophage activation during Mycobacterium tuberculosis infection and leads to enhanced protection in the absence of IFN-γ signaling. J Immunol 2016;197:4714-26. [Crossref] [PubMed]
- Gerez L, Madar L, Shkolnik T, et al. Regulation of interleukin-2 and interferon-γ gene expression in renal failure. Kidney Int 1991;40:266-72. [Crossref] [PubMed]
- Mansouri L, Nopp A, Jacobson SH, et al. Hemodialysis patients display a declined proportion of Th2 and regulatory T cells in parallel with a high Interferon-γ profile. Nephron 2017;136:254-60. [Crossref] [PubMed]
- Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58:470-80. [Crossref] [PubMed]
- Kaufmann SHE, Weiner J, von Reyn CF. Novel approaches to tuberculosis vaccine development. Int J Infect Dis 2017;56:263-7. [Crossref] [PubMed]
- Van Der Meeren O, Hatherill M, Nduba V, et al. Phase 2b Controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 2018;379:1621-34. [Crossref] [PubMed]



