
Adjuvant treatment can improve prognosis in patients with non-small cell lung cancer ≤3 cm after sublobectomy: a propensity score analysis
Introduction
Lung cancer is one of the most common malignancies worldwide. With the application of low-dose spiral computed tomography (CT), an increasing number of small lung nodules have been detected, thus leading to an increase in the discovery of early-stage lung cancer (1). According to the current National Comprehensive Cancer Network (NCCN) guidelines, the recommended treatment for early-stage lung cancer is surgery, which includes lobectomy and sublobectomy. Because the prognosis of sublobectomy is poor, lobectomy with mediastinal lymph node dissection or sampling is preferred, while sublobectomy and segmentectomy can be chosen if the patient cannot tolerate lobectomy. Although adjuvant therapy is not recommended for early-stage lung cancer (2), sublobectomy cannot provide accurate staging, this part of patients which don’t know accurate staging after sublobectomy cannot be considered as early lung cancer (3).
Since 1995, a series of studies have shown that the prognosis of sublobectomy is worse than lobectomy (4,5). In 1995, Ginsberg and Rubinstein conducted a study on the effects of lobectomy and sublobectomy on survival in early-stage lung cancer, reporting that sublobectomy led to worse outcomes in patients than lobectomy (4). The reasons behind this finding were not clearly explained, though it may be linked to insufficient lymph node dissection and insufficient distance from the resection margin. Furthermore, in many sublobectomies, the number of lymph nodes removed is insufficient or the lymph nodes are not removed (5). Liu et al. showed that more than half of the patients had less than 6 lymph nodes dissected during sublobectomy (3).
There are no clear criteria for the selection of suitable patients for sublobectomy, so some patients may have good prognosis after undergoing sublobectomy (6), whilst others may have poor prognosis. Additionally, there is no relevant research exploring how to improve the prognosis of patients. Hamada et al. showed that adjuvant chemotherapy significantly improves survival in patients with stage IA T1b non-small cell lung cancer (NSCLC) compared with surgery alone (7). Based on these considerations, we speculated that adjuvant therapy may improve the prognosis of these patients. Therefore, the purpose of this study was to determine whether radiotherapy and chemotherapy could improve the prognosis of patients undergoing sublobectomy.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3448).
Methods
The clinical data of patients who were pathologically diagnosed with NSCLC between 2004 and 2015 were extracted using the SEER*Stat software from the Surveillance, Epidemiology, and End Results (SEER) database. The eligibility criteria were as follows: 20 years of age or older, underwent sublobectomy, and NSCLC tumor ≤3 cm. The collected data included age, gender, pathological type, stage, tumor diameter, number of lymph node dissections, surgical methods, radiotherapy and chemotherapy, overall survival (OS), lung cancer-specific survival (LCSS). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software. Comparisons of the basic characteristics of tumors between groups were performed using a χ2 test and t-test. The Kaplan-Meier method was used for univariate survival analysis. The Cox proportional hazards model was used for multivariate analysis. A P value of <0.05 was considered statistically significant.
Results
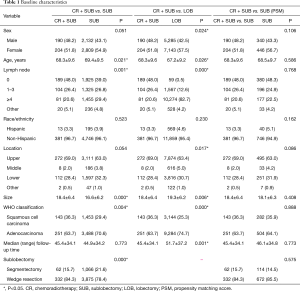
There were 17,763 eligible NSCLC cases found in the SEER database. Lobectomy was performed in 12,428 cases, and sublobectomy was performed in 5,335 cases. In the sublobectomy group, among the 394 patients treated with adjuvant therapy, 202 were treated with radiotherapy, 50 with chemoradiotherapy, and 142 with chemotherapy. The median follow-up time for lobectomy was 51.7 months, the median follow-up time for sublobectomy alone was 44.9 months, and the median follow-up time for sublobectomy with chemoradiotherapy was 45.4 months. Larger tumor diameter, lower number of lymph node dissections, and more wedge resections were observed in the patients treated with adjuvant therapy. No significant differences were found in regard to sex, ethnicity, and median (range) follow-up time between the two groups (Table 1).
Full table
After propensity score matching of the sublobectomy patients with and without adjuvant therapy using a ratio of 1:2, there were 394 patients in the adjuvant therapy group and 786 patients in the surgery group. The median follow-up time in the adjuvant therapy group was 45.4 months, and the median follow-up time in the surgery group was 46.1 months (Table 1).
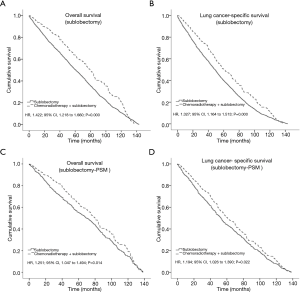
In the subsequent survival analysis, the OS of adjuvant therapy patients showed a significant survival advantage over those treated with sublobectomy alone [hazard ratio (HR), 1.422; 95% CI, 1.218 to 1.660; P=0.000] (Figure 1A). In terms of LCSS, there were significant differences in survival between the adjuvant treatment group and the surgery-only group (P<0.05; Figure 1B). The survival analysis was performed again after the propensity match scoring, generating similar results for OS (HR, 1.251; 95% CI, 1.047 to 1.494; P=0.014) (Figure 1C) and LCSS (P<0.05; Figure 1D).
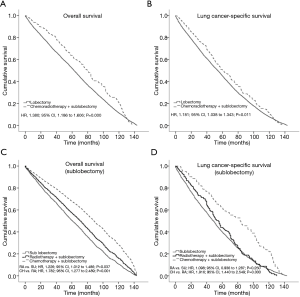
There was still a significant difference in OS between adjuvant therapy and lobectomy alone (HR, 1.380; 95% CI, 1.186 to 1.606; P=0.000) (Figure 2A). In terms of LCSS, there were significant differences in survival between the adjuvant treatment group and the surgery-only group (P<0.05; Figure 2B). When chemotherapy, radiotherapy, and chemoradiotherapy were compared with sublobectomy alone, the survival benefit of adjuvant therapy was more obvious than that of surgery-only (P<0.05; Figure 2C,D), and chemotherapy was more beneficial than radiotherapy and chemoradiotherapy.
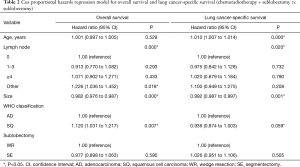
The Cox proportional hazards regression model showed that the factors that could affect OS in the sublobectomy versus chemoradiotherapy group were the number of lymph node dissections, tumor pathological types, and tumor size (P<0.05, Table 2). The correlation factors after propensity match scoring were the number of lymph node dissections and tumor size (P<0.05, Table 3). In the comparison between lobectomy and chemoradiotherapy, the factors influencing OS were the number of lymph node dissections, tumor pathological type, and tumor size (P<0.05, Table 4). In terms of LCSS, the factors affecting prognosis in the sublobectomy and chemoradiotherapy groups were age, number of lymph node dissections, and tumor size (P<0.05, Table 2). Age was also an influencing factor after propensity match scoring (P<0.05, Table 3). The relevant factors affecting prognosis in the lobectomy versus chemoradiotherapy groups were age, and number of lymph node dissections (P<0.05, Table 4).
Full table
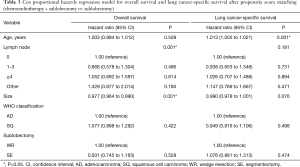
Full table
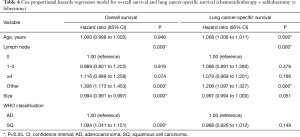
Full table
Discussion
Half a century ago, the debate among surgeons for lung cancer patients was whether to choose lobectomy or pneumonectomy (8). Nowadays, the focus of debate among surgeons for patients with operable clinical stage I NSCLC is whether to choose lobectomy or sublobectomy. The factors influencing prognosis are surgical method, margin, tumor diameter, number of lymph node dissections, and pathological type for operable NSCLC. In order to find a suitable standard for sublobectomy, some studies have explored the diameter of the tumor (9), the number of lymph nodes dissected (3), and the pathological type (10).
Despite the increased detection rate of pulmonary nodules, the choice of surgical method for patients with stage I NSCLC is controversial in the field of thoracic surgery. The current NCCN guidelines recommend lobectomy for stage Ia NSCLC, however, sublobectomy should still be recommended for patients with stage I NSCLC who cannot tolerate lobectomy (2). Consequently, there are no clear criteria for the selection of patients suitable for sublobectomy, which may lead to poor prognosis in some patients due to inappropriate surgical methods.
In a randomized controlled study on sublobectomy and lobectomy from 1995, lobectomy was superior to sublobectomy in terms of survival and local recurrence (4). However, some thoracic surgeons believe this study has many shortcomings, such as an insufficient margin, erroneous pathological classification, and lymph nodes that were not dissected. The margin is also an essential factor that should be taken into consideration after sublobectomy of NSCLC. The adequate margin of wedge resection should ideally be >2 cm or at least 1 cm to reduce local recurrence. However, it has been reported that wedge resection is frequently associated with margins less than 1 cm (48–61%). Even in segmentectomy, 23% to 27% of presented margins are less than 1 cm (11,12). Insufficient margin has been associated with a high risk of locoregional recurrence, which may partially account for sublobectomy failure. Nowadays, surgical methods are much better than before. Although there are still no randomized controlled studies, numerous retrospective studies have reported inconsistent results (13). In particular, segmentectomy can be used to successfully achieve anatomic resection. However, the results of previous studies may not necessarily be applicable today, and sublobectomy, which is similar to lobectomy, can be performed in some patients, especially on ground-glass opacity (GGO) nodules (6,14). Although many studies have reported good prognosis after sublobectomy for GGO nodules (15,16), Moon et al. reported that sublobectomy in clinical N0 solid-predominant nodules could actually increase recurrence due to the marginal factors (13).
Some studies have suggested that tumor diameter is related to the type of surgery, meaning that sublobectomy could be performed on tumors smaller than 20 mm in diameter (17,18). Chen et al. demonstrated that the difference in survival between sublobectomy and lobectomy was not statistically significant when the diameter was less than 2 cm (5). Several retrospective studies have shown that limited resection may be equally as effective for the treatment of stage Ia patients with tumors ≤2 cm compared with lobectomy, particularly among elderly patients (4,6,19). Kates et al. analyzed the outcomes of patients with NSCLC ≤1 cm, and concluded that lobectomy conferred no OS benefit (20). However, other studies have found that tumor diameter cannot be used as a criterion for choosing surgical methods because lymph node metastasis can occur even if the tumor diameter is very small (9). For example, when the tumor diameter is less than 20 mm, the rate of N1 and N2 lymph node metastasis can be as high as 5.3% and 6.6% (21). It is well known that sublobectomy may understage lung cancer because of inadequate lymphadenectomy for hilar (N1) lymph nodes compared with lobectomy (22). Yendamuri et al. demonstrated that the survival advantage offered by lobectomy over sublobectomy in NSCLC patients with tumor size ≤2 cm has incrementally decreased over the past 2 decades with advancements in surgical methods, however, the prognosis of lobectomy is still better than sublobectomy (23). Therefore, for many NSCLC cases, although the tumor is less than 3 cm in size, it cannot be identified as stage I lung cancer as the lymph node is not guaranteed to be negative after sublobectomy. In order to obtain accurate staging, intraoperative systemic lymph node dissection is required. In the current study, lung adenocarcinoma was the main pathological type in the sublobectomy group. According to the newly revised classification of pulmonary adenocarcinoma by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS), adenocarcinoma is divided into adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive adenocarcinoma (24). There is no lymph node metastasis in the presence of adenocarcinoma in situ or minimally invasive adenocarcinoma, resulting in 100% 5-year disease-free survival (10), whereas those with invasive adenocarcinoma have 76–84% 5-year disease-free survival (25). Many thoracic surgeons believe that sublobectomy can be performed for adenocarcinoma in situ and minimally invasive adenocarcinoma. Nonetheless, lobectomy should be performed for invasive adenocarcinoma (25,26). At present, preoperative examinations, such as bronchoscopy and CT-guided percutaneous lung biopsy, can only be recommended for patients with pathological types and cannot be further classified due to the small number of samples. While intraoperative freezing can obtain enough tissue to quickly determine the pathological type, there is a certain error in intraoperative freezing pathology, which may lead to the erroneous diagnosis of some invasive adenocarcinoma such as adenocarcinoma in situ, thus leading to inappropriate surgical treatment (27).
This study showed that sublobectomy had worse prognosis than lobectomy, hence, sublobectomy is not recommended for all ≤3 cm NSCLC. We verified that sublobectomy may not perform adequate lymph node dissection, and some lung cancers with longer diameters also received sublobectomy. Therefore, we believe that the poor prognosis of some patients is due to not being given the correct surgery. Although the SEER database does not explain why adjuvant therapy was given, the present study showed that the prognosis of these patients significantly improved after adjuvant therapy, reaching even more optimal results than lobectomy. Moreover, other studies have also confirmed that radiotherapy and chemotherapy demonstrate efficacy in stage I patients (28,29).
The present study has some limitations that need to be addressed. First, the SEER database does not provide specific chemotherapy regimens and radiation doses. Second, this study is a retrospective study. Although the propensity score matching analysis was performed, there is still a possibility of some bias. Finally, due to the low number of chemotherapy, radiotherapy, and chemoradiotherapy cases, they were all grouped into the 1 same group to reduce bias, which could not be analyzed in more detail.
In summary, the results of this study show that chemoradiotherapy can improve the OS of patients after sublobectomy and reduce death caused by tumors. Therefore, when patients cannot tolerate lobectomy or are given inappropriate surgery, adjuvant therapy can improve the prognosis of patients. Our conclusion still needs to be verified using prospective randomized controlled trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3448
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3448). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Liu Y, Shen J, Liu L, et al. Impact of examined lymph node counts on survival of patients with stage IA non-small cell lung cancer undergoing sublobar resection. J Thorac Dis 2018;10:6569-77. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Yu Y, Huang R, Wang P, et al. Sublobectomy versus lobectomy for long-term survival outcomes of early-stage non-small cell lung cancer with a tumor size ≤2 cm accompanied by visceral pleural invasion: a SEER population-based study. J Thorac Dis 2020;12:592-604. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Hamada C, Tsuboi M, Ohta M, et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 2009;4:1511-6. [Crossref] [PubMed]
- Faber LP. Individual ligation technique for lower lobe lobectomy. Ann Thorac Surg 1990;49:1016-8. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer</= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Kent M, Landreneau R, Mandrekar S, et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg 2013;96:1747-54; discussion 1754-5. [Crossref] [PubMed]
- Moon Y, Lee KY, Moon SW, et al. Sublobar Resection Margin Width Does Not Affect Recurrence of Clinical N0 Non-small Cell Lung Cancer Presenting as GGO-Predominant Nodule of 3 cm or Less. World J Surg 2017;41:472-9. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; discussion 762-4. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (</= 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Witt C. European respiratory society/american thoracic society/international association for the study of lung cancer international multidisciplinary classification of lung adenocarcinoma: state of the art. J Thorac Oncol 2011;6:1451. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Xie D, Wang TT, Huang SJ, et al. Radiomics nomogram for prediction disease-free survival and adjuvant chemotherapy benefits in patients with resected stage I lung adenocarcinoma. Transl Lung Cancer Res 2020;9:1112-23. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


