
Early identification of patients with severe COVID-19 at increased risk of in-hospital death: a multicenter case-control study in Wuhan
Introduction
On March 11th 2020, the World Health Organisation (WHO) announced the coronavirus (COVID-19) pandemic (1). By late June 2020, more than 9,000,000 cases had been confirmed and approximately 400,000 deaths reported globally (2). Although most cases to date are classified as mild, with the rapid spread of COVID-19 and dramatic increase in confirmed cases, the 14% of cases that are severe and 5% that are critical have overwhelmed intensive care units (ICUs) and health care capacity around the globe; this has resulted in an in-hospital mortality rate of over 50% (3,4). Early recognition and rational allocation of ICU resources for severe cases at high risk of poor outcome after admission are therefore of great importance in reducing the mortality rate and rationalizing health care resources.
To date, there have been many reports on this topic. However, previous studies developing early triage or warning systems for severe COVID-19 patients have mostly focused on laboratory data at the time of hospital admission but few have examined the trajectory of this data during the hospitalization (5,6). Furthermore, according to a recent report on BMJ, the relevant importance of underlying health conditions is unclear due to inadequate adjustment for key confounding factors, such as age, sex, etc. (7). We, therefore, explore the dynamic changes in laboratory parameters during the first 10 days after admission to allow early detection of these cases. We conducted a multicenter, aged matched case-control study in an attempt to more efficiently control the confounding effect of age, the most commonly reported confounding factor, for a better understanding of underlying risk factors. The objective was to delineate the clinical characteristics of patients with severe COVID-19 who are at increased risk of in-hospital death.
We present the following article/case in accordance with the STROBE Checklist (available at http://dx.doi.org/10.21037/jtd- 20-2568).
Methods
Study design and setting
This multicenter, aged matched case-control study was conducted between January 2020 and March 2020 in Jinyintan hospital, Wuhan Pulmonary Hospital and Sino-French New City Branch of Tongji Hospital, which were among the government designated hospitals for providing care to patients with severe COVID-19 in Wuhan, China. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Commission of Tongji Hospital (TJ-IRB20200326). Informed consent was waived by the Ethics Commission due to the rapid spread of this infectious disease.
Study population
Patients admitted to the study hospitals between January 1 and February 23, 2020 were included if meeting the following inclusion criteria: (I) aged 18 years and over; (II) diagnosed as having severe COVID-19 according to the Guidelines for the Diagnosis and Treatment of Corona Virus Disease (8); To receive this diagnosis patients had one or more of the following: respiratory distress with respiratory rate >30 breaths/min, oxygen saturation (SpO2) <93% on room air, or arterial oxygen index (arterial partial pressure of oxygen/fraction of inspired oxygen, PaO2/FiO2) <300 mmHg. Laboratory confirmation of COVID-19, SARS-CoV-2 RNA detection by real time-PCR, was performed by the local health authority as previously described (9); (III) had a definitive outcome, deceased or discharged, during the study period. The discharge criteria were according to National Health Commission Guidelines (8) are: absence of fever for at least 3 days, clinical remission of respiratory symptoms, substantial improvements of both lungs in chest CT, negative test for SARS-CoV-2 RNA for two consecutive respiratory samples (sampling at least 24 hours apart). Patients were excluded if an alternative medical diagnosis explained their clinical presentations or had missing outcome and baseline information. Non-survivors and survivors were matched 1:1 according to age ±5 years, in an attempt to more efficiently control the confounding effect of age at the design stage. Clinical outcomes were monitored up to March 3, 2020.
Previous study reported the prevalence of elevated D-dimer (≥0.5 mg/L) among survivors infected with SARS-CoV-2 in Wuhan was 47.9%, and elevated D-dimer was associated with higher risk of mortality [odds ratio (OR): 3.47, 95% confidence interval (CI): (1.15, 10.48), P value: 0.027] compared with low D-dimer (<0.5 mg/L) (10). Assuming a power of 95% and a two-sided 0.05 significance level, the sample size of this matched case-control study was calculated as 174 patients (87 pairs) using PASS software version 11.0.
Variables
We collected data on demographics (age, gender), medical history, signs and symptoms, laboratory findings, medical treatment (including oxygen therapy, antiviral agents, antibiotics, corticosteroids, vasoconstrictive agents, immunoglobulin, interferon, thymosin, continuous renal replacement therapy, and extracorporeal membrane oxygenation) and outcomes. Fever was defined as axillary temperature over 37.3 °C. Secondary infection was diagnosed when patients had clinical signs or symptoms of pneumonia or bacteremia and a positive culture of a new pathogen obtained from blood samples or lower respiratory tract specimens after admission (9). Shock, acute respiratory distress syndrome (ARDS) and sepsis were defined according to the WHO interim guidelines (11). Acute renal injury was, defined according to clinical practice guideline (12). Acute cardiac injury was diagnosed if hypersensitive cardiac troponin I was above the 99th percentile of the upper reference limit (9). Acute liver failure was diagnosed according to AGA guideline (13).
Data collection
Data were collected from electronic medical records onto a standardized data collection form modified from the standardized International Severe Acute Respiratory and Emerging Infection Consortium case report form (11). A team of physicians was educated on the use of this form. Data were collected on admission days 1, 4, 7 and 10. Clinicians from participating hospitals identified patients who met the inclusion criteria through screening all patients in their units during the study period. The research team included the physicians who provided direct care to patients with COVID-19 in the study hospitals during the study period, ensuring identification of all cases meeting the inclusion criteria. The diagnostic definitions of severe COVID-19 were recorded separately and checked by a senior member of the research team.
Data were independently entered and cross checked by the researchers and a team of trained physicians. Any ambiguous or missing data were clarified and collected by communication with treating physicians who provided direct patient care. Patients’ confidentiality was maintained by removing personal identifiers on the data collection form.
Statistical analysis
Continuous and categorical variables were summarized as median (interquartile range) and frequencies (percentages). To compare the differences between survivors and non-survivors, the Mann-Whitney U test was used for continuous variables, and χ2 test or Fisher’s exact test for categorical variables. Univariate and multivariate conditional logistic regression models were used to explore the risk factors associated with in-hospital mortality; associations were reported using the odds ratio (OR) and 95% confidence intervals (CI). We estimated parameters of collinearities and finally selected representative laboratory variables from different physiological systems based on expert consensus and previous literature (14). Lymphocyte count, C-reactive protein, D dimer, aspartate aminotransferase, and oxygenation index were included as independent variables in the multivariate model adjusted for gender and hypertension. For regression analyses, multiple imputation was performed, based on the Markov chain Monte Carlo method, to impute missing values of sequential organ failure assessment (SOFA) score and laboratory data. Differences in laboratory variables between days 1, 4, 7, 10 were compared by Friedman test, which is appropriate for rank test of continuous variables in repeated measurement designs. Temporal trends of laboratory data between groups were compared using mixed linear models with residual maximum likelihood. A two-sided P<0.05 was considered statistically significant. Data management and analyses were conducted using R version 3.6.1.
Results
Characteristics of the studied population
We enrolled 157 non-survivors and 213 survivors during the study period (Figure S1). Of these, 64 were excluded due to missing outcome information (n=25), baseline information (n=3), or laboratory data (n=36). The final analysis included 93 non-survivors and 93 survivors matched for age (±5 years). Of the 186 patients, the median age was 68.0 (62.0, 74.0) years, 119 (64.0%) were male, and 118 (63.4%) had a comorbidity. Hypertension was the most common comorbidity, followed by diabetes and coronary heart disease in both groups (Table 1). Fever was the most common presenting symptom on admission in both groups. The second most common symptom for survivors was cough (67, 72.0%) and dyspnea (73, 78.5%) for non-survivors. Compared with non-survivors, survivors were more likely to be male, have a history of hypertension, with onset symptoms of sputum production, myalgia, fatigue and dyspnea (Table 1). Non-survivors had a higher SOFA score (4.0 (3.0, 7.5)) than survivors [2.0 (1.0, 3.0)] (Table 1).
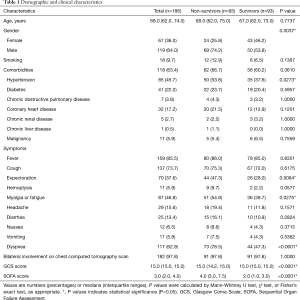
Full table
The median time from onset of symptoms to hospital admission was 10.0 (7.0, 13.0) days for survivors and 10.0 (7.0, 14.0) days for non-survivors (Table 2). After hospital admission, compared with survivors, non-survivors were more likely to receive certain medical treatment including antibiotics [91 (97.9%) vs. 67 (72.0%)], nasal cannula oxygen [90 (96.8%) vs. 74 (79.6%)], invasive mechanical ventilation [74 (79.6%) vs. 3 (3.2%)], and glucocorticoids [72 (77.4%) vs. 59 (63.4%)] (Table 2). The most commonly observed complications in non-survivors included ARDS (81, 87.1%), secondary infection (55, 59.1%), acute cardiac injury (51, 54.8%) and shock (47, 50.5%). The only complication observed in survivors was ARDS (44, 47.3%) (Table 2). Detailed information on treatments and outcomes for survivors and non-survivors are shown in Table 2.
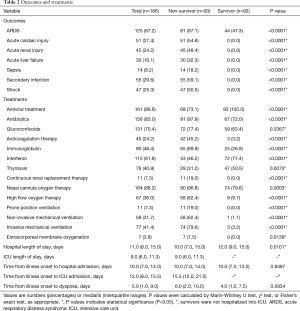
Full table
The dynamic changes of laboratory parameters during the first 10 days of hospitalization
Laboratory data were tracked at admission days 1, 4, 7 and 10 (Tables 3 and 4). The overall trends of 10 laboratory parameters differed significantly between non-survivors and survivors (P value of group, and/or time <0.05; Figure 1). The overall trends of other 11 laboratory parameters (including brain natriuretic peptide, cardiac troponin I, creatine kinase, ect.) were indicated in Figure S2. In particular, a persistent remarkably higher CRP concentration was observed in non-survivors, but a time-dependent decreasing trend among survivors in the first 10 days after admission. The median lymphocyte count dropped to 0.4×109/L in non-survivors on day 4 and being unmitigated thereafter. Among survivors median lymphocyte count recovered from 0.8×109/L to 1.0×109/L. From day 4, non-survivors were prone to develop thrombocytopenia (<100×109/L) and deteriorating thereafter, with a median platelet count approximately half the value of survivors whose platelet count progressively returned to normal range. The medians of D-dimer in survivors never exceed 1.4 mg/L. In non-survivors, d-dimer had a sharp increase [21.0, (9.2, 21.0)] on day 4 and then fell to 13.9 (4.2, 21.0) on day 7. Compared to survivors who had an increased oxygen index (OI) in a time-dependent manner, non-survivors had poor blood oxygenation characterizing by deteriorating OI even after receiving oxygen therapy. In addition, among non-survivors, hypoalbuminemia was not alleviated even after continuous immunoglobulin infusion.
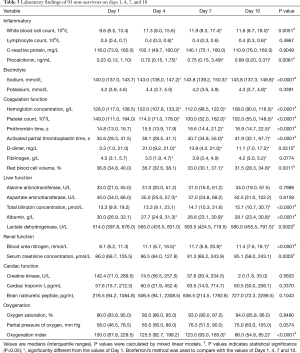
Full table
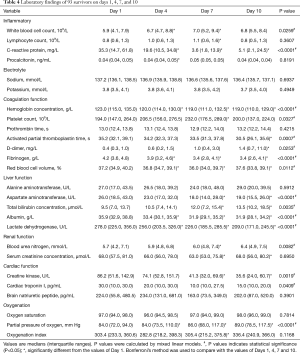
Full table
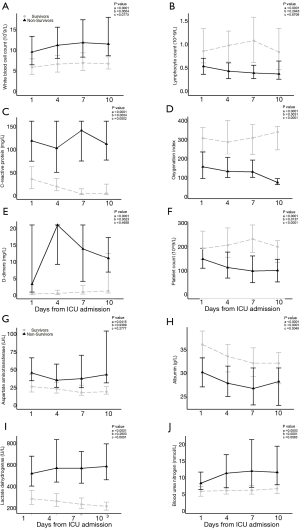
Risk factors associated with in-hospital mortality
Compared with survivors (Table 5), on admission non-survivors had more severe lymphopenia (lymphocyte count <0.6×109/L), uncontrolled inflammatory responses (C-reactive protein >100 mg/L), hyper-fibrinolysis (D-dimer >1 mg/L; platelet count ≤100×109), poor blood oxygenation [oxygen index (OI) ≤200], multiple organ injuries (indicated by notably higher biomarkers for acute cardiac, renal and liver injury) and refractory hypoalbuminemia (albumin <32 g/L).
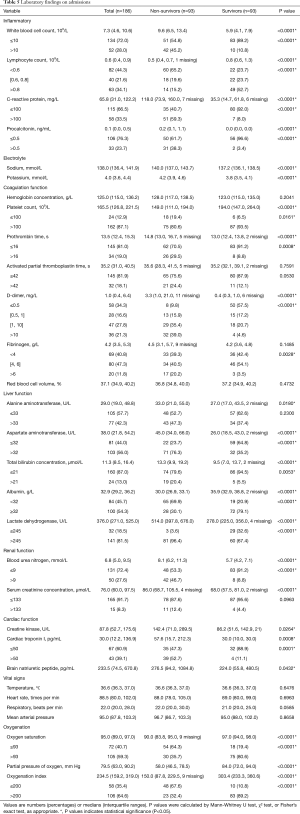
Full table
In univariable analysis (Figure 2), male gender, hypertension, symptoms (including sputum production, fatigue and dyspnea), SOFA score, WBCs, lymphocyte count, CRP, procalcitonin, platelet count, D-dimers, aspartate aminotransferase (AST), total bilirubin (TB), albumin, serum creatinine, cardiac troponin I (cTnI) and OI, were associated with increased odds of in-hospital mortality (P<0.05, Figure 2). Seven independent variables were selected (see statistical analysis) for multivariate analysis. After adjusting for confounding factors, five laboratory parameters were independent risk predictors of in-hospital death for patients with severe COVID-19: severe lymphopenia (≤0.6×109/L), CRP (>100 mg/L), D-dimer (>1.0 mg/L), AST (>32 U/mL) and OI (≤200). We further performed multiple logistic regression analyses for risk factors stratified by sex or hypertension. The associations of risk factors with in-hospital mortality were similar across patients with and without hypertension (Table S1). The sample sizes for subgroup in female, might be too small to detect statistical differences in mortality between different gender groups.
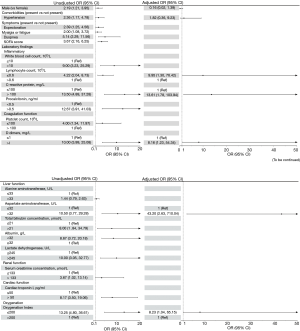
Discussion
In this multicenter, age matched, case-control study, several mortality risk factors were identified for patients hospitalized with severe COVID-19. On admission, patients characterized with severe lymphopenia (lymphocytes count <0.6×109/L), uncontrolled inflammatory responses (CRP >100 mg/L), hyper-fibrinolysis (D-dimer >1 mg/L), poor blood oxygenation (oxygen index ≤200), liver injury (aspartate aminotransferase >32 U/L) had increased risk of in-hospital death. After admission, sharp increases in D-dimer at day 4 but decreasing lymphocyte and platelet count from day 4, deteriorating oxygen index, and persistent remarkably high CRP concentration, were early warning signs of poor outcome.
Consistent with previous literature, in univariate analysis, we observed that male patients with SARS-CoV-2 infection had a higher odds risk of in-hospital death (15,16). Murine models have shown that endogenous estrogens reduce virus replication in female nasal epithelial cells and restrict pulmonary pro-inflammatory cytokines in the context of influenza virus infection (17,18). Males with unhealthier lifestyles, such as smoking and alcohol consumption, and poorer awareness of their health status (16,19), are at increased risk of death if they contract COVID-19. Consistent with other research, we also found that patients with severe COVID-19 who have a history of hypertension are at increased risk of in-hospital mortality (20). Patients with hypertension are often treated with ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), both of which would upregulate the expression of ACE2 in lung tissues and blood vessel endothelium (21). Increased ACE2 serves as negative regulator in the renin-angiotensin system (RAS) and produces vasodilatation effects by targeting angiotensin II, but also facilitates SRAS-CoV-2 entry and replication in luminal epithelium.
In the acute phase of viral pneumonia, rapid increase in CRP concentrations indicates acute infection which parallels the severity of inflammation or tissue injury (22). It assists complements and phagocytes to eliminate the virus by binding to infected cells (23). The serum concentration of CRP generally peaks in 48 hours and quickly comes down to a lower level once virus induced inflammation subsides (8). A recent study suggested that a CRP greater than 100 mg/L could be an important prognostic indicator of 30-day mortality among patients with sepsis (24).
Lymphopenia is another striking characteristic of patients with severe COVID-19 on admission (25). The counts of CD4+/CD8+ T cells and CD19+ B cells are reportedly reduced in patients with SARS-CoV-2 infection, because SARS-CoV-2 directly generates an immunosuppressive effect on the myeloid cells which were confirmed to express ACE2 and these immune cells are recruited to clear the viral infection (26,27). Sustaining functional exhaustion of circulating lymphocytes means persistent local inflammation in target organs and strongly suggests severe multiple organ injury. In our study this was evidenced by elevated WBCs, procalcitonin, AST, lactate dehydrogenase, blood urea nitrogen and serum creatinine during the early phase of hospitalization of patients with severe COVID-19 who subsequently died.
A constant decline in OI from 150 on day 4 to below 100 on day 10 from admission suggested one hallmark of uncontrolled inflammatory response or cytokine storm in the lungs, and development of ARDS and respiratory dysfunction in these patients. Hence, decreases in these laboratory findings and failure to normalize in the first days of hospitalization suggest disease progression in severe patients with COVID-19.
Additionally, 65.7% of patients in our study presented with elevated levels of D-dimer, a fibrin degradation product. Increases in blood concentration of D-dimer, commonly caused by sepsis or hypoxemia, indicate hyper-fibrinolysis or suspected thrombotic disorders and is an important predictive indicator of venous thromboembolism or acute pulmonary embolism (28). From day 4 of hospitalization, sharp increases in D-dimer, accompanying decreasing platelet counts and prolonged prothrombin times in 80.3% (110/137) of severe cases in our study (particularly in non-survivors) highlighted a hypercoagulable state. This eventually leads to disseminated microembolization in multiple organs as reported by recent autopsy (29). Typically, micro emboli formation in small branches of the pulmonary artery in turn affects internal respiration, resulting in the exacerbation of hypoxemia and respiratory failure (30). Extrapulmonary microembolization, particularly in the coronary artery, has been identified as a potential cause of acute cardiac injury, which is a common complication in severe COVID-19 patients (31). Even worse, more than half of the patients with severe COVID-19 and a remarkable elevation in D-dimer progressed quickly into septic shock in a short time in this study. Evidence in the current study thus suggests a positive correlation between elevated D-dimer in the early disease stage and a poorer prognosis for COVID-19.
Combining with the results from risk factor analysis using conditional logistic regression models, these aforementioned indicators (lymphocyte and platelet count, CRP, D-dimer and OI) significantly differ between survivors and non-survivors. Furthermore, during the first 4-7 days of hospitalization, dramatic alterations in these laboratory findings in male patients with severe COVID-19 and pre-existing hypertension, were early warning signs of increased risk of in-hospital death.
Several limitations need to be acknowledged. Firstly, the sample size is relatively small, though we collected all patients admitted to the three study hospitals between January 1 and February 23, 2020, the most serious pandemic period in China. Among 213 survivors enrolled in this study, 93 were matched with non-survivors and entered final analysis. We admitted that we lost some survivor patients during the matching process in an attempt to more efficiently control the confounding effect of age at the design stage. Secondly, some laboratory tests (for example fibrinogen, and arterial blood gas) were not performed on all patients; missing data might lead to bias in clinical characteristics between cohorts. Thirdly, our study participants only included severe cases of COVID-19, leading to limited generalizability to cases with mild symptoms. Fourthly, several potential confounders including health-related behaviors and severity of comorbidities were not collected in the medical records. In addition, the detailed treatment history for COVID-19 before admission, which has a significant impact on adverse clinical events, was unavailable especially for those patients who were transferred at a late stage of their illness to the study hospitals.
Conclusions
Better understanding of the clinical features of patients with severe COVID-19 during the early stage of hospitalization is vital for timely identification of those at risk of poor outcomes, since laboratory data from illness onset to hospital admission is often not clinically available. Severe illness rapidly progresses to death within a short time of hospitalization in these patients. The predictive utilities of dynamic changes in lymphocyte count, CRP, D-dimer and OI can contribute to early recognition of COVID patients at high risk of in-hospital mortality and with early supportive care, creates the potential for improved outcomes. These findings may be helpful for clinicians’ early triage for severe COVID-19 patients who are at increased risk of in-hospital death.
Acknowledgments
We are grateful to all patients and medical staff involved in this study.
Funding: The research was funded by grants from the Huazhong University of Science and Technology COVID-19 Project (2020kfyXGYJ087).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at http://dx.doi.org/10.21037/jtd-20-2568
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2568
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2568). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-JRB20200225). Written informed consent was waived due to the rapid spread of the epidemic.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Djalante R, Shaw R, DeWit A. Building resilience against biological hazards and pandemics: COVID-19 and its implications for the Sendai Framework. Progress in Disaster Science 2020;6:100080 [Crossref]
- Organization WH. Coronavirus disease 2019 (COVID-19): Situation Report, 72. 2020.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Organization WH. Coronavirus disease 2019 (COVID-19): situation report, 41. 2020.
- Sun Q, Qiu H, Huang M, et al. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Annals of Intensive Care 2020;10:33. [Crossref] [PubMed]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [Crossref] [PubMed]
- Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ 2020;368:m1198. [Crossref] [PubMed]
- Lin L, Li T. Interpretation of "guidelines for the diagnosis and treatment of novel coronavirus (2019-ncov) infection by the national health commission (trial version 5)". Zhonghua Yi Xue Za Zhi 2020;100:E001 [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2. Eur Respir J 2020;56:2002961 [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [PubMed]
- Flamm SL, Yang YX, Singh S, et al. American Gastroenterological Association Institute Guidelines for the Diagnosis and Management of Acute Liver Failure. Gastroenterology 2017;152:644-7. [Crossref] [PubMed]
- Desai MY, Smedira NG, Dhillon A, et al. Prediction of sudden death risk in obstructive hypertrophic cardiomyopathy: Potential for refinement of current criteria. J Thorac Cardiovasc Surg 2018;156:750-9.e3. [Crossref] [PubMed]
- Liu N, Zhang F, Wei C, et al. Prevalence and predictors of PTSS during COVID-19 Outbreak in China Hardest-hit Areas: Gender differences matter. Psychiatry Res 2020;287:112921 [Crossref] [PubMed]
- Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8:e20 [Crossref] [PubMed]
- Finch CL, Zhang A, Kosikova M, et al. Pregnancy level of estradiol attenuated virus-specific humoral immune response in H5N1-infected female mice despite inducing anti-inflammatory protection. Emerging Microbes Infections 2019;8:1146-56. [Crossref] [PubMed]
- Jones BG, Sealy RE, Penkert RR, et al. From Influenza Virus Infections to Lupus: Synchronous Estrogen Receptor α and RNA Polymerase II Binding Within the Immunoglobulin Heavy Chain Locus. Viral Immunol 2020;33:307-15. [Crossref] [PubMed]
- von Bothmer MI, Fridlund B. Gender differences in health habits and in motivation for a healthy lifestyle among Swedish university students. Nurs Health Sci 2005;7:107-18. [Crossref] [PubMed]
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531-8. [Crossref] [PubMed]
- Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020;27:taaa041.
- Coster D, Wasserman A, Fisher E, et al. Using the kinetics of C-reactive protein response to improve the differential diagnosis between acute bacterial and viral infections. Infection 2020;48:241-8. [Crossref] [PubMed]
- Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. WMJ 2016;115:317-21. [PubMed]
- Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J Crit Care 2020;56:73-9. [Crossref] [PubMed]
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020;17:533-5. [Crossref] [PubMed]
- Thevarajan I, Nguyen TH, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 2020;26:453-5. [Crossref] [PubMed]
- Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135-140. [Crossref] [PubMed]
- Chen J, Wang X, Zhang S, et al. Findings of Acute Pulmonary Embolism in COVID-19 Patients. Available at SSRN 3548771 2020.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Xie Y, Wang X, Yang P, et al. COVID-19 Complicated by Acute Pulmonary Embolism. Radiology: Cardiothoracic Imaging 2020;2:e200067 [Crossref]
- Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol 2020;5:831-40. [Crossref] [PubMed]


