
A nomogram for predicting brain metastases of EGFR-mutated lung adenocarcinoma patients and estimating the efficacy of therapeutic strategies
Introduction
Lung cancer, a type of malignant tumor, is known the world over for having the highest morbidity and mortality of all cancers. Accounting for 80–85% of lung cancer cases is non-small cell lung cancer (NSCLC) (1). Brain metastases (BMs) are the main form of distant metastases in lung cancer and one of the common reasons for treatment failure. Approximately 25% of patients with NSCLC suffer from BM, which influences their survival. Tailored individual survival estimates could prove useful for advising patients with BM on treatment-related decisions and enhancing approaches to therapy. In early 1997, RTOG put forward the classification of RPA (recursive partitioning analysis) (2), which was the first prognostic scoring system for assessing the prognosis of patients with BM. In 2008, Sperduto et al. (3) published the Graded Prognostic Assessment (GPA), a new prognostic score system. Individual cancer prognoses are regularly estimated using nomograms (4-9), in the main as they are able to transform statistical predictive models into numerical forms to produce an estimation for the probability of death, recurrence, or another event, taking into consideration the individual’s profile (10). Although nomograms are widely used for cancer prognosis (4-9), this approach has not been applied to EGFR-mutated lung adenocarcinoma patients with BM.
NSCLC patients with BMs have poor prognosis despite therapies, with a 1-year survival rate of less than 20% (3). WBRT (whole-brain radiation therapy) serves as a standard treatment for NSCLC patients with BM, which has resulted in an OS in the range of 3 to 6 months since the 1970s (11,12). Tyrosine kinase inhibitor (TKI), a small molecule with a good lipid-water partition coefficient, can be absorbed with little difficulty and has high permeability; therefore, it is able to pass through the cell membrane and the BBB (blood brain barrier). EGFR mutations are key targets in predicting TKI treatment efficiency for NSCLC patients. More recently, TKI therapy has been explored for brain metastatic patients with EGFR mutations with an effective rate of approximately 70–80% (13). A Japanese research team reported that among 41 patients, the complete and partial response rate was 87.8% (14). Furthermore, the intracranial progression free survival time was 14.5 months and the overall survival (OS) was 21.9 months. For almost half of the subjects, radiation therapy was delayed by TKI for over 1.5 years after being diagnosed with BM (14). Accordingly, some experts pointed out that TKI targeted therapy has become a favorable treatment, especially for patients with EGFR mutation of BMs of lung cancer, challenging the status of radiotherapy as a result. However, for individuals with EGFR-mutated NSCLC who have BM, the optimal treatment is yet to be established (15), and this important question remains to be examined.
This study was conducted with the purpose of developing a nomogram model to estimate survival probabilities for individual patients with EGFR-mutated lung adenocarcinoma with BM. We developed a nomogram based on clinical features for predicting prognosis and the value of radiotherapy and TKI for treating the patients with different prognosis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1587).
Methods
Study design and patient eligibility
We collected data from patients who had stage I–IV lung cancer treated at our hospital from January 1st 2011 to December 31st 2014. Patients were included in this study if they were pathologically confirmed to have adenocarcinoma and genetically confirmed to have EGFR mutations in exon 19 and/or exon 21.The patients with meningeal metastases were excluded because of the poor prognosis.
A total of number of 560 cases were initially selected. We analyzed and summarized the 129 (23.0%) cases who had brain parenchymal metastases at initial diagnosis or developed BMs during routine follow-up as the first progression. The patients were followed up by hospitalization and/or outpatient clinic consultations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the independent Ethics Committee of Tianjin Medical University Cancer Institute & Hospital and individual consent for this retrospective analysis was waived.
Treatment schedule
Diagnosis of BM was made based on an enhanced head MRI. After the diagnosis of BM, 106 (82.3%) patients received treatments including radiotherapy, chemotherapy, and targeted therapy. Radiotherapy included whole brain radiotherapy (WBRT, 40 Gy/20 f or 30 Gy/10 f) and/or stereotactic radiotherapy. Cisplatin-based chemotherapy was taken by the chemotherapy group. The targeted therapy treatment medicines were Gefitinib (250 mg, oral, once per day), Erlotinib (150 mg, oral, once per day), or Icotinib (125 mg, oral, 3 times per day). The oral medicines were taken until the disease progressed, or an unacceptable adverse reaction or death occurred.
Statistical analyses
OS was defined as the period of time from diagnosis to death or last follow-up. The Kaplan-Meier method was applied to conduct survival analysis, and log-rank test was used to calculate comparisons between groups. Univariate and multivariate analyses were carried out using Cox proportional hazard models. Statistical significance was considered to exist when P value <0.05. The nomogram was constructed based on the Cox proportional hazards regression model for survival data. The nomogram score was verified by way of receiver operating characteristic (ROC) curves and internal calibration blots. Kaplan-Meier and Cox analysis were carried out using SPSS 18.0 software; and R software, version 3.2.2, was used for the nomogram, ROC, and internal validation.
Results
Patient characteristics
Of the included cases, 50 cases were male (38.9%) and 79 cases were female (61.1%). The median age of onset was 58, and 80.5% of the patients were ≤65 years. Approximately 36% of the cases were smokers. Regarding mutations, approximately 42% of the cases carried exon 19 mutation and 58.0% of the cases carried exon 21 mutation. The number of patients with stages I–III and stage IV lung adenocarcinoma at initial diagnosis were 110 (85.3%) and 19 (14.7%), respectively (Table 1).
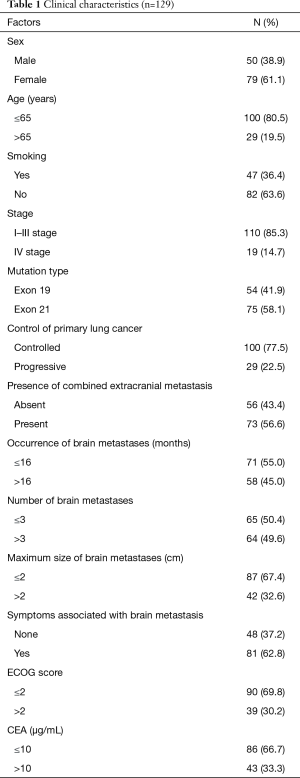
Full table
Kaplan-Meier curves
The median OS of all patients was 36 months, and the 1- and 2-year survival rates were 91.5% and 72.4%, respectively. The median OS after BM diagnosis was 16 months, and the 1- and 2-year survival rates were 55.1% and 46.5%, respectively.
The median follow-up time for the whole group was 28 months, and the follow-up rate was 100%.
Univariate and multivariate results
In the univariate analysis, the stage, ECOG score, interval between the diagnoses of lung cancer and BM, the number of brain metastatic lesions, and the diameter of the maximal brain metastatic lesion correlated well with OS. In the multivariate Cox proportional hazard analysis, the ECOG score, interval between the diagnoses of lung cancer and BM, and the number of brain metastatic lesions were the significant prognostic factors related to OS (Table 2).
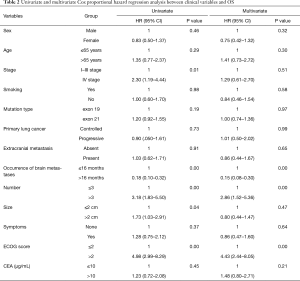
Full table
Nomogram
The nomogram we developed was based on the significant risk factors (P<0.05) identified using multivariate analysis. Stage had P<0.01 in the univariate analysis, and it was also taken into account in the nomogram. The nomogram for predicting OS was built based on stage, ECOG score, interval between the confirmed diagnoses of lung cancer and BM, and the number of brain metastatic lesions (Figure 1). The nomogram was used make predictions of the 2- and 3-year OS probabilities; Figure 2 displays associated ROC curves and internal calibration plots. The concordance index for the model was 0.83. The nomogram-predicted survival was well calibrated with the Kaplan-Meier observed survival.


Nomogram scores to predict the efficacy in the subgroup analysis
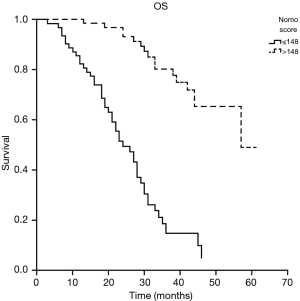
Risk scores for the patients were calculated based on the nomogram model. The median score of the nomogram was 157 [0–257]. Based on ROC analysis, a cutoff value of 148 was chosen for OS prediction, and the patients were split into two groups, the poor prognostic group (score: 0–148) and the good prognostic group (score >148) based on this cutoff value. Kaplan-Meier analysis revealed that median OS were 24 months in poor prognostic group and 57 months in good prognostic group (P<0.05). The 1- and 2-year survival rates in the poor prognostic group were 82.3% and 49.4%, respectively. The 1- and 2-year survival rates for the good prognostic group were 100% and 93.2%, respectively (P<0.05) (Figure 3).
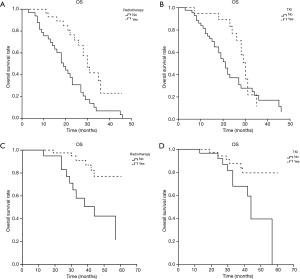
Subgroup K-M analysis was conducted to compare the two treatments: radiotherapy and TKI. In the poor prognostic group, patients who received radiotherapy have longer OS than the patients who received no radiotherapy for the first-line treatment (30 vs. 19 months, P<0.05). The OS was 30 months in TKI subgroup and 21 months in no TKI group, which has no statistical difference (P>0.05). In good prognostic group, patients who received radiotherapy have a higher 3-y OS rate than the patients who received no radiotherapy for the first-line treatment (91.2% vs. 58.1%, P<0.05). The 3-y OS rate was 87.6% in TKI subgroup and 67.8% in no TKI group, which has a statistical difference (P<0.05) (Figure 4).
Discussion
Many patients with lung cancer develop BMs, which impacts quality of life and dramatically decreases survival. Up to 40% of those who receive an NSCLC diagnosis will go on to develop BMs at some point over the course of their disease; a risk which could be even greater for those who harbor EGFR exon mutations (15). Previous studies have found EGFR mutations to be significantly associated with increased risk of BM (16,17) and the distinct clinical profiles of EGFR-mutated tumors in relation to BM have been pointed out (18-21). Therefore, it is speculated that BMs in these patients exhibit their own characteristics in occurrence, treatment, and prognosis.
The results of our univariate analysis showed stage, ECOG score, interval between the diagnoses of lung cancer and BM, and the number and diameter of BMs lesions to be correlated with OS. The multivariate Cox proportional hazard analysis showed that the ECOG score, interval between the confirmed diagnoses of lung cancer and BM, and the number of BMs lesion were the prognostic factors significantly associated with OS. Previous studies concluded that the performance status (2,11,22,23), age (2,11,22,23), extracranial metastases (2,11,22,23), and primary tumor control (11,16) have a potential effect on survival. Other studies (24,25) indicated that the number of brain metastases influences survival. None of the previous studies were targeted on patients with BMs with EGFR-mutated lung adenocarcinoma. Most of the patients in our study were treated with TKI, which could have potentially impacted our results that differed from previous studies.
When compared with traditional prognostic indicators, nomograms may provide prognostic estimates which are tailored to individual patients to a greater degree. The predictive and prognostic models were subjected to internal validation, and they were indicated by calibration and discrimination tests to perform well. Previous studies were focused on the patients with BM (26-29). Our study is the first to develop a nomogram for patients with BM with EGFR-mutated lung adenocarcinoma. Our Kaplan-Meier analysis revealed that the median OS and the 1- and 2-year survival rates in the high nomogram group were better than in the low nomogram group (P<0.05). As such, the nomogram may prove useful for assessing risk as well as selecting therapies according to the patient as an individual.
In the past, surgical resection, stereotactic radiosurgery (SRS), or whole-brain radiotherapy, either individually or in combination, have been employed to treat patients with BMs. Tyrosine kinase inhibitor (TKI), a small molecule with a good lipid-water partition coefficient, can be absorbed with little difficulty and has high permeability; therefore, it is able to pass through the cell membrane and the BBB. Some experts have pointed out that TKI targeted therapy is becoming a favorable treatment, especially for patients with EGFR-mutated lung cancer who have of BMs (13,14), while others (30,31) believed that radiotherapy is irreplaceable for these patients. A meta-analysis showed that EGFR-TKIs used by itself should be the first-choice treatment for NSCLC patients who have multiple BM, particularly those with EGFR mutation, as it offers similar OS and extracranial PFS but superior intracranial PFS in comparison with WBRT plus EGFR-TKIs (31). A recent study suggested that in a selective patient group, SRS and TKI could offer, when used in combination, an effective form of treatment for those with BM who have favorable brain control and little neurotoxicity (32). A new study showed that for patients with EGFR-mutated NSCLC who develop BMs, the upfront administration of EGFR-TKI and deferral of radiotherapy are related to poorer OS. EGFR-TKI following SRS saw the longest OS for patients, allowing them to evade the potential neurocognitive sequelae of WBRT (33). In conclusion, the optimal treatment for EGFR-mutated NSCLC patients with BM 2 is unknown, and this is an important research issue which needs to be addressed. Patients who have different prognoses may require different individualized approaches to treatment. Our research divided patients into two groups, the poor prognostic group and the good prognostic group. Our research indicated that in poor prognostic group, patients who received radiotherapy had a longer OS than the patients who did not receive radiotherapy as the first-line treatment (30 vs. 19 months, P<0.05). The OS was 30 months in the TKI subgroup and 21 months in the no TKI group, and there was no statistical significance (P>0.05). In the good prognostic group, patients who received radiotherapy had a better 3-y OS rate than the patients who did not receive radiotherapy as the first-line treatment (91.2% vs. 58.1%, P<0.05). The 3-y OS rate was 87.6% in the TKI subgroup and 67.8% in the no TKI group, and there was statistical significance (P<0.05). We then concluded that radiotherapy was beneficial for all patients, whatever their prognosis, but TKI may be more suitable for the patients who had a better prognosis. In the first 24 months after BM, the prognosis of the TKI subgroup looked better than that of the no TKI subgroup in the poor prognostic group, but after 24 months, the OS curve of the TKI subgroup dropped rapidly. Drug resistance is a likely reason for this. Patients who had a poor prognosis may have a poor performance, more or larger brain metastatic lesions, so it was probably lack of salvage treatment if the drug resistance occurs.
Conclusions
The nomogram was useful for predicting BMs in EGFR-mutated lung adenocarcinoma patients and for selecting individualized therapies. Radiotherapy was beneficial for all the patients, whatever the prognosis, but TKI may be better suited for treating patients who have a better prognosis.
Limitations
There are certain limitations to this study. First, this study is a retrospective analysis focusing on a relatively small amount of cases and a limited follow-up duration. Therefore, there were differences in the primary treatments patients underwent, and these treatments might have affected the survival assessment. This study therefore cannot offer a comprehensive reflection of prognosis of BMs in patients in relation to this. Improved data collection and/or a randomized controlled study are necessary to explore in-depth answers for these research questions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1587
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1587
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1587). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the independent Ethics Committee of Tianjin Medical University Cancer Institute & Hospital and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qin H, Wang C, Jiang Y, et al. Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery: a systematic review. Med Sci Monit 2015;21:144-52. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis(RPA) of prognostic factors in three Radiation Therapy Oncology Group(RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 2008;26:380-5. [Crossref] [PubMed]
- Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol 2014;32:3939-47. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Van Zee KJ, Patil S. Validation of a nomogram for predicting risk of local recurrence for ductal carcinoma in situ. J Clin Oncol 2012;30:3143-4. [Crossref] [PubMed]
- Iasonos A, Schraq D, Rai GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Diener-West M, Dobbins TW, Phillips TL, et al. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys 1989;16:669-73. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine inhibitors for brain metastases in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Lu Y, Fan Y. Combined action of EGFR tyrosine kinase inhibitors and whole-brain radiotherapy on EGFR-mutated non-small-cell lung cancer patients with brain metastases Onco Targets Ther 2016;9:1135-43. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastases in pulmonary adenocarcinoma. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Lee YJ, Park IK, Park MS, et al. Activating mutations within the EGFR kinase domain: a molecular predictor of disease-free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol 2009;135:1647-54. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival; after diagnosis of brain metastases in non small cell lung cancer. Neuro Oncol 2010;12:1193-9. [Crossref] [PubMed]
- Enomoto Y, Takada K, Hagiwara E, et al. Distinct features of distant metastases and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig 2013;51:153-7. [Crossref] [PubMed]
- Gerosa M, Nicolato A, Foroni R, et al. Analysis of long-term outcomes and prognostic factors in patients with non-small cell lung cancer brain metastases treated by gamma knife radiosurgery. J Neurosurg 2005;102 Suppl:75-80. [Crossref] [PubMed]
- Zindler JD, Rodrigues G, Haasbeek CJ, et al. The clinical utility of prognostic scoring systems in patients with brain metastases treated with radiosurgery. Radiother Oncol 2013;106:370-4. [Crossref] [PubMed]
- Rades D, Schild SE, Lohynska R, et al. Two radiation regimens and prognostic factors for brain metastases in non-small cell lung cancer patients. Cancer 2007;110:1077-82. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit. Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Rotin DL, Paklina OV, Kobiakov GL, et al. Lung cancer metastases to the brain: Clinical and morphological prognostic factors. Zh Vopr Neirokhir Im N N Burdenko 2013;77:24-8. [PubMed]
- Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol 2012;14:910-8. [Crossref] [PubMed]
- Park Y, Kim KS, Kim K, et al. Nomogram prediction of survival in patients with brain metastases from hepatocellular carcinoma treated with whole-brain radiotherapy: a multicenter retrospective study. J Neurooncol 2015;125:377-83. [Crossref] [PubMed]
- Omuro AMP, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with non small cell lung carcinoma after response to gefitinib. Cancer 2005;103:2344-8. [Crossref] [PubMed]
- Zheng H, Liu QX, Hou B, et al. Clinical outcomes of WBRT plus EGFR-TKIs versus WBRT or TKIs alone for the treatment of cerebral metastatic NSCLC patients: a meta-analysis. Oncotarget 2017;8:57356-64. [Crossref] [PubMed]
- Yang WC, Xiao F, Shih JY, et al. Epidermal growth factor receptor mutation predicts favorable outcomes in non-small cell lung cancer patients with brain metastases treated with stereotactic radiosurgery. Radiother Oncol 2018;126:368-74. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective MultiInstitutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]


