A comparison of non-intubated video-assisted thoracic surgery with spontaneous ventilation and intubated video-assisted thoracic surgery: a meta-analysis based on 14 randomized controlled trials
Introduction
At present, traditional endotracheal intubation with general anesthesia is a widely accepted method in thoracoscope surgery. Utilizing this procedure, patients receive one-lung ventilation, which provides a stable surgical field and operating space for the thoracoscope surgery (1). However, endotracheal intubation with general anesthesia is inevitably associated with a risk of complications such as airway hyperresponsiveness, postoperative sore throat, hoarseness, and pulmonary inflammatory reactions (2-4).
In recent years, the concept of “enhanced recovery after surgery” (ERAS) has been widely promoted, and non-intubated video-assisted thoracic surgery (NIVATS) has been increasingly used to avoid injury associated with tracheal intubation and the residual effects of muscle relaxants, thereby promoting the recovery of the patient’s postoperative respiratory function and sputum discharge function, and reducing the occurrence of postoperative complications (5-8). This technique is currently being used in pleural effusion, spontaneous pneumothorax, empyema resection, wedge resection, lung volume reduction, thymectomy, segmentectomy, and lobectomy (9-12). However, complications such as intraoperative cough, mediastinal oscillation, hypercapnia, and hypoxemia remain unresolved and may require surgeons’ more delicate and stable operation and improved anesthesia management. Also, laryngeal mask airway (LMA) inflation can cause a feeling of pharyngeal compression, and as a result, some patients may experience postoperative pharyngeal pain, pharyngeal nerve compression injury, and other deficiencies (13). Therefore, the implications of NIVATS are still not fully understood and remain controversial.
To date, there is a lack of a large sample, multi-center, and high-quality evidence to demonstrate the safety and efficacy of NIVATS and to determine whether it allows for an acceptable surgical field. Furthermore, it is unclear whether patients undergoing NIVATS experience shorter hospital stays and fewer postoperative complications such as pharyngeal discomfort and hoarseness. Therefore, in this study, operation time and surgical field satisfaction scores were used to evaluate the operation’s safety from the surgeon’s perspective. Short-term postoperative pain was measured by anesthesia satisfaction score and visual analogue scale (VAS) score (24 hours after surgery). The occurrence of complications evaluated postoperative rehabilitation quality, and the length of hospital stay was assessed to determine whether NIVATS was beneficial to the overall rehabilitation of patients. This information will be beneficial for the surgical decision-making process. We present the following article in accordance with the PRISMA reporting checklist (available at
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations were adopted for this investigation (14). This study is currently being registered with PROSPERO, and a registration number is pending. A systematic and comprehensive computer search was conducted using the Cochrane Library, Web of Science, PubMed, Embase, and ClinicalTrials.gov databases to screen for randomized controlled trials (RCTs) up to August 2020 by combining search terms such as “non-tracheal intubation”, “non-intubated”, “wake”, “video-assisted thoracoscopic surgery”, “VATS” and “thoracic disease”. There were no restrictions on the year or country of publication. Also, manual filtering was performed on the list of references from the original articles and review articles to retrieve studies not detected in the database search.
Inclusion and exclusion criteria
Eligible studies were included according to the following criteria: (I) RCTs comparing non-intubated versus intubated general anesthesia in thoracic surgery; (II) the presences of sufficient data to conduct a study of mean differences (MDs) or odds ratio (OR); (III) both groups of patients in the study underwent thoracoscopic surgery; and (IV) the most recent study was selected in case of duplication. Exclusion criteria were as follows: (I) no comparison between non-intubation thoracoscopy and endotracheal intubation thoracoscopy; (II) intubated and non-intubate patients underwent different surgical procedures; (III) reviews, letters, editorials, expert opinions, case reports, and animal experiments, and (IV) a failure to extract relevant data from the study.
Data extraction
The authors used standard tables to extract data from the included studies independently. RCTs comparing the efficacy of NIVATS and video-assisted thoracoscopic surgery (VATS) in the treatment of thoracic diseases were searched. The two authors resolved any differences through discussion, and the corresponding author ultimately decided any disputes that could not be resolved.
Validity assessment
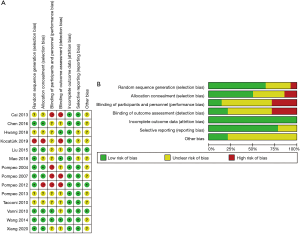
The Jadad scale was used to evaluate the quality of the selected publications, with a score of 3 or above defined as a high-quality study. The Cochrane collaboration bias risk assessment tools were used to evaluate the quality of the RCTs, and this involved the following seven aspects: (I) random sequence generation (selection bias); (II) allocation concealment (selection bias); (III) blinding of participants and personnel (performance bias); (IV) blinding of the outcome assessment (detection bias); (V) incomplete outcome data (attrition bias); (VI) selective reporting (reporting bias), and (VII) other bias. All aspects were evaluated according to “low bias”, “unclear”, and “high bias”.
Statistical analysis
RevMan 5.3 software was used for meta-analysis, and the effect indicators were MD, 95% confidence interval (CI) for quantitative data, and OR and 95% CI: for qualitative data. Data were considered statistically significant when P<0.05. The heterogeneity of treatment effects between studies was measured using Higgins’ inconsistency test (I2). If I2 ≤50%, the heterogeneity was accepted, and the fixed effects model was selected. If I2 >50%, then heterogeneity was considered to be large. In this case, the source of heterogeneity was searched, and subgroup analysis, sensitivity analysis, or the random effects model was used for meta-analysis. The median and quartile range of continuous variables were converted to mean standard deviation using the sample mean estimation method (15) and the standard deviation estimation method (16) by using the online tool (
Results
Search results
The basic characteristics of the included studies
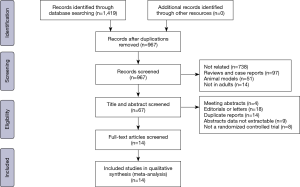
A total of 14 RCTs were included in the study according to the PRISMA guidelines (shown in Figure 1), including 1,426 patients, with 707 patients in the non-intubated group and 719 patients in the intubated group (Table 1).

Full table
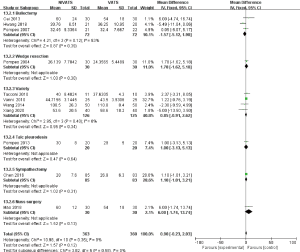
As shown in Table 1, based on the Jadad scale, eight studies were of good quality (18,19,21-23,25,29,30) with scores of 3 or above. The types of surgery in these reports are mostly classified as thoracoscopic operations. Bullectomy (17,19,24), wedge resection (23), sympathectomy (18), talc pleurodesis (26), and pleural biopsy (20) are defined as minor thoracic surgery. Nuss surgery (22) and lung volume reduction surgery (25) are defined as moderate thoracic surgery. Lobectomy and segmentectomy (30) are defined as major thoracic surgery. The basic characteristics of the included studies are shown in Table 1.
Sensitivity analysis and bias risk assessment
Risk assessment of bias was conducted according to the bias risk assessment tool recommended by the Cochrane system. Most of the studies included in this paper described random methods, allocation concealment, blindness, and data integrity. There were nine studies (18,21-24,28-30) with a low risk of selection bias, using random numbers generated by the computer. A total of two studies (18,29) used the sealed envelope method, and four reports (22-24,28) used the opaque random sequence method. In terms of measurement bias, three studies (19,22,29) used the method of third-party measurement collection. However, some studies did not mention the bias risk assessment table, and the quality of the methodology was poor. The quality evaluation results of the included studies are shown in Figure 2.
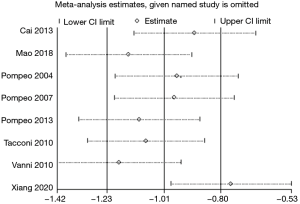
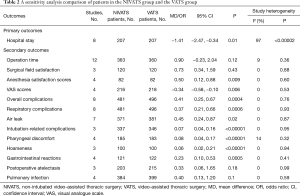
Sensitivity analysis was performed by successively removing each of the included studies from the overall analysis. The results demonstrated that the other analyses’ initial results were not altered except for postoperative air leakage, indicating that most of the results were stable (Table 2).
Full table
Meta-analysis results
Primary outcome
A total of eight articles (17,22-24,26-28,30) reported the length of hospital stay, but the heterogeneity was vast (P<0.00001, I2 =97.0%). Subgroup analysis was conducted according to operation size, anesthesia mode, and ethnicity; however, each study’s heterogeneity was still large (Figures 3–6). Sensitivity analysis showed that the MD deviated from the original 95% CI: after eliminating the study by Xiang et al. (30) (Figure 7), suggesting that this study was the source of heterogeneity. The latter study’s surgical methods included lobectomy and segmental lung resection, and the postoperative hospital stay was longer than that of other minor and moderate operations. The random effects model demonstrated that the length of hospital stay in the non-intubated group was significantly shorter compared to patients with intubation (MD −1.41; 95% CI: −2.47 to −0.34; P=0.01; Figure 3). The publication bias test was conducted, and both the Egger’s and Begg’s tests revealed no obvious publication bias (P=0.127 and P=0.902, respectively).
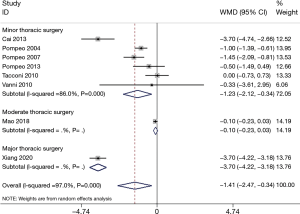
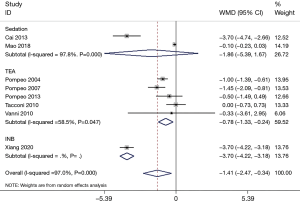
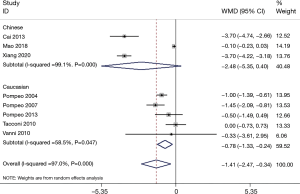
Secondary outcomes
Operation time
A total of 12 studies (17-20,22-24,26-30) reported the operation time, and the heterogeneity was large (P<0.0001, I2 =81%). Sensitivity analysis showed that the heterogeneity was reduced to the acceptable range (P=0.36, I2 =9%) after excluding the report by Kocatürk et al. (20). Unilateral and bilateral pleural biopsies were the main procedures examined in this study, explaining the significant difference in operation time. After exclusion, the remaining studies were combined with the fixed effects model, and this revealed that the operation time of non-intubated thoracoscopic surgery was not statistically different compared to patients with intubation (MD 0.90; 95% CI: −0.23 to 2.03; P=0.12). Subgroup analysis was carried out for the other 11 studies. No significant differences were observed compared to the original analysis (Figure 6). The funnel plot analysis showed that the operation time results are distributed symmetrically (Figure 8).
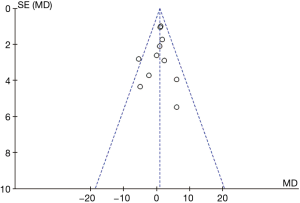
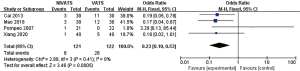
Surgical field satisfaction (1 point)
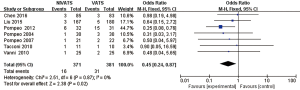
Surgical field satisfaction (1 point) was reported in three studies (17,29,30) with small inter-study heterogeneity (P=0.88, I2 =0%). A surgical field satisfaction of 1 point denotes complete lung collapse with a well-exposed operative field. A score of 2 points denotes normal lung collapse with a relatively clear surgical field of vision, with no need to interrupt the operation. A score of 3 points represents a poor surgical field exposure with unsatisfactory lung collapse, necessitating repeated interruption to the surgery. A score of 4 points represents poor exposure to the surgical field and a failure to complete the operation, necessitating the transfer to intubation surgery. In all three studies that reported the surgical field satisfaction, the patients’ surgical field satisfaction was mostly 1 (complete lung collapse with a well-exposed operative field). The fixed effects model demonstrated that the difference in surgical field satisfaction between the NIVATS group and the VATS group was not statistically significant (OR 0.73; 95% CI: 0.34 to 1.59; P=0.43; Figure 9).

Anesthesia satisfaction scores
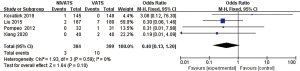
Anesthesia satisfaction scores were reported in four studies (23,24,26,27), with small inter-study heterogeneity (P=0.6, I2 =0%). The fixed effects model revealed that anesthesia satisfaction scores in the NIVATS group were significantly higher than the VATS group (MD 0.50; 95% CI: 0.12 to 0.88; P=0.009; Figure 10).
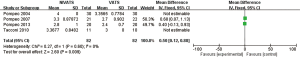
VAS score
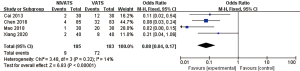
The VAS scores were reported in four studies (19,20,23,26), with little inter-study heterogeneity (P=0.53, I2 =0%). The fixed effects model showed that the VAS scores in the NIVATS group were significantly lower than those in the VATS group (MD −0.34; 95% CI: −0.58 to −0.10; P=0.006; Figure 11).

Complications
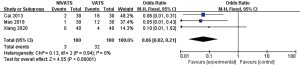
The incidences of overall complications (OR 0.41; 95% CI: 0.25 to 0.67; P=0.0004), respiratory complications (OR 0.37; 95% CI: 0.21 to 0.66; P=0.0006) including air leakage (OR 0.45; 95% CI: 0.24 to 0.87; P=0.02), intubation-related complications (OR 0.07; 95% CI: 0.04 to 0.16; P<0.00001) including pharyngeal discomfort (OR 0.08; 95% CI: 0.04 to 0.17; P<0.00001) and hoarseness (OR 0.06; 95% CI: 0.02 to 0.21; P<0.00001), and gastrointestinal reactions (OR 0.23; 95% CI: 0.10 to 0.53; P=0.0005) were significantly lower in the NIVATS group compared to the VATS group. The incidences of postoperative atelectasis (OR 0.33; 95% CI: 0.06 to 1.65; P=0.18) and pulmonary infections (OR 0.40; 95% CI: 0.13 to 1.20; P=0.10) in the NIVATS group were not significantly different from those in the VATS group. All studies demonstrated small heterogeneity (P>0.01, I2 =0%) and the fixed effects model was used for analysis (Figures 12-20).
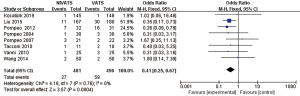
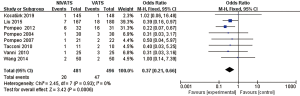


Discussion
A total of 1,426 patients were included in 14 RCTs to evaluate the safety and efficacy of NIVATS. The random effects model analysis showed that the hospitalization time of patients in the NIVATS group was shorter than that of patients in the VATS group. However, the heterogeneity between studies was large, and this could not be resolved by using multiple subgroup analyses. This could be due to the large variety of diseases and surgical procedures presented in these studies, the small sample size, and the large variations in the length of hospital stay in each study. In addition to the type of operation, the postoperative chest-tube dwell time and the application of antibiotics can also affect hospital stay length. Furthermore, a patient’s discharge may depend on the subjective assessment of a patient’s rehabilitation status. Therefore, further large-scale studies are required to support the results of this meta-analysis.
This report’s investigations demonstrated that there was no significant difference in the operation time and surgical field satisfaction between patients in the NIVATS group and the VATS group. However, in terms of the operation time, there was considerable heterogeneity among studies. Considering the variety of surgical procedures in the included studies, it cannot simply be assumed that the operation time in NIVATS patients would not be longer than that in VATS patients. Despite the risks of intraoperative mediastinal oscillation, cough, and insufficiency of lung collapse (31), the study showed that the surgeon’s subjective satisfaction score with field conditions did not decrease in NIVATS. In the three studies that reported surgical field satisfaction, most of the patients in both groups had an surgical field satisfaction of 1 point, and no cases with poor surgical field conditions were reported. In recent years, serratus anterior block and spinal horizontal block have emerged as effective methods for reducing intraoperative pain (32). A degree of diaphragmatic movement and mediastinal movement is acceptable, although excessive mediastinal movement may affect surgical procedures (33). The length of the patient’s operation time was not prolonged due to the improved surgical field conditions. This suggested that the selection of patients who strictly conform to the indications (34,35), combined with precise intraoperative anesthesia management and the surgeon’s gentle and skilled operation, can achieve a surgical field experience comparable to that of conventional endotracheal intubation VATS.
Patients in the NIVATS group showed higher anesthesia satisfaction scores, which indicated that flexible and stable anesthesia management during NIVATS surgery enables patients to obtain better sedative and analgesic effects. Also, the pain associated with tracheal intubation and the residual effects of muscle relaxants were avoided, which may explain the lower VAS scores in patients in the NIVATS group than patients in the VATS group 24 hours after surgery.
In this study, the incidence of total complications in the NIVATS group was lower than that in the VATS group. Additionally, the incidences of respiratory complications (including postoperative pneumothorax, atelectasis, and pulmonary infection), intubation-related complications (including pharyngeal discomfort and hoarseness), and digestive tract reactions were lower in the non-intubated group compared to the intubated group. The differences in the complication indexes were all statistically significant, except for atelectasis and pulmonary infection. A sensitivity analysis of postoperative air leakage was conducted. After removing the publication by Pompeo et al. (25), the initial results (P=0.02) were altered (P=0.25). However, the pneumothorax incidence in the NIVATS group was still lower than that in the VATS group, but the difference was not statistically significant. An analysis of the literature included in this study revealed a high incidence of air leakage in emphysema patients who had undergone lung volume reduction surgery. Mechanical ventilation with endotracheal intubation can result in pulmonary barotrauma, and regional hyperventilation can lead to alveolar rupture (36). Also, the opening and closing of the terminal bronchi and alveoli with ventilator-mediated ventilation can result in shear stress on lung tissue cells, namely, shear force injury. NIVATS avoids mechanical ventilation and can effectively reduce the incidence of pneumothorax after lung volume reduction surgery (37). However, with the exception of Liu et al. (21) and Pompeo et al. (25), there were no significant differences in the incidences of total complications and respiratory complications between NIVATS patients and VATS patients. This suggested that NIVATS mainly reduced the occurrence of hoarseness and pharyngeal discomfort, with no obvious advantages in air leakage, atelectasis, and other aspects. It is interesting to note that the VATS group experienced a high incidence of postoperative pharyngeal pain. Therefore, particular attention should be given to standardized endotracheal intubation requirements and to explore novel methods and techniques to reduce patient discomfort.
Colonized bacteria in the pharynx and larynx can enter the lower respiratory tract with tracheal intubation, causing opportunistic respiratory infections and postoperative symptoms such as sore throat, cough, and sputum (38). Also, residual muscle relaxants can delay the recovery time of patients’ cough and sputum ability after surgery, leading to a series of complications such as postoperative atelectasis (39). A prospective study showed that the incidence of postoperative pharyngeal pain and hoarseness due to endotracheal intubation was 44% (40). However, Puri et al. (41) believed that inflation of the LMA capsule could cause a feeling of pharyngeal compression, and hence some patients may also experience postoperative pharyngeal pain, pharyngeal nerve compression injury, and other deficiencies. Our meta-analysis showed that the incidence of total intubation-related complications, pharyngeal discomfort, and hoarseness in the NIVATS group was lower than that in the VATS group, suggesting that non-intubation reduced postoperative pharyngeal pain and hoarseness.
Other studies (42) have shown that muscle relaxants significantly increase postoperative nausea and vomiting, leading to a high incidence of postoperative gastrointestinal reactions by reducing intestinal perfusion and oxygen delivery. Furthermore, systemic opioid analgesics can also inhibit gastrointestinal function. The data from this meta-analysis support this.
Previous meta-analyses showed that patients undergoing non-intubated thoracoscopic surgery had shorter hospital stays and postoperative fasting (43), less postoperative inflammation, and better immune function recovery (44). However, most of the included studies were retrospective studies, and the selection and reporting bias may have affected the study results. In the current meta-analysis, all included literature were RCTs to minimize the selection bias as much as possible. After the risk assessment of bias in all the literature, it was noted that most of the literature applied randomized grouping and blind methods, and therefore the risk of bias was lower.
The safety and efficacy of non-intubation thoracic surgery were assessed from the surgeon and the patient’s perspective. The surgical environment of NIVATS was evaluated from the surgeon’s perspective by the surgical field score, and the operation time was objectively measured. The anesthesia satisfaction score evaluated short-term postoperative pain, and the VAS score 24 hours after surgery. Indicators, including pharyngeal discomfort, hoarseness, and postoperative air leakage, were analyzed to evaluate the patients’ perioperative rehabilitation quality. Combined with the overall length of hospital stay, the results demonstrated that NIVATS was beneficial to the patients’ overall rehabilitation. However, a number of RCTs reported on anatomical resections such as lobectomy and segmentectomy. Therefore, further large-scale, high-quality clinical randomized trials are warranted.
Despite the advantages, NIVATS has certain shortcomings, such as intraoperative hypoxemia and hypercapnia, mediastinal oscillation, and cough reflex, and therefore some researchers have suggested that the value of NIVATS should be re-examined. As a novel method of anesthesia, NIVATS has stricter patient indications. Numerous studies have demonstrated that NIVATS avoids the complications of VATS and accelerates the patient’s postoperative rehabilitation. It may indeed be a new option for patients who cannot tolerate endotracheal intubation, such as patients with neck trauma requiring immobilization or patients with severe cervical spondylosis undergoing elective surgery. Further extensive research is warranted to understand the benefits and risks associated with NIVATS fully.
Conversion of NIVATS to VATS has been reported in two studies. Hung et al. (45) suggested that patients in NIVATS should be immediately transferred to tracheal intubation anesthesia in the following situations: (I) respiratory acidosis where pH <7.1; (II) hypoxemia (PO2 <60 mmHg) with no improvement following high-flow oxygen inhalation and non-invasive ventilation; (III) continuous cough with no improvement following aerosolized lidocaine and vagus nerve block; (IV) anxiety attack and invalid sedation; (V) voluntary conversion of patients; and (VI) intraoperative massive hemorrhage. The long-term survival of NIVATS patients is also of concern. In 2012, Pompeo et al. reported that the rates of freedom from contralateral treatment in group NIVATS and VATS were 55% versus 50%, and survival rates were 81% versus 87% at 36 months. It will be beneficial for future investigations to examine long-term survival in NIVATS patients.
There are some limitations to this report. First, in some studies included in this investigation, risk bias evaluation factors such as random method, blind method, allocation, and hiding were not clearly described, which may affect the final research conclusion’s authenticity. Second, due to the small number of included studies, the heterogeneity could not be reduced by subgroup analysis, and the heterogeneity was still large. Third, indications and contraindications were lacking in multi-center large sample prospective clinical studies, and therefore, the long-term benefits are not clear.
Conclusions
NIVATS can significantly reduce intubation-related complications, relieve postoperative pharyngeal and gastrointestinal discomfort, and reduce patients’ postoperative VAS scores. NIVATS is a technology co-created by the thoracic surgery department and the anesthesiology department to pursue a “holistic minimally invasive strategy” and “ERAS”. The aim is to minimize postoperative clinical management pressure and greatly reduce the patient’s postoperative pain. To date, several studies have demonstrated that NIVATS is safe for use in a variety of chest diseases and may be an important development in the field of minimally invasive thoracic surgery in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3039).
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-3039
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3039). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elkhayat H, Gonzalez-Rivas D. Non-intubated uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2019;11:S220-2. [Crossref] [PubMed]
- Bahk JH. Guidelines for determining the appropriateness of double-lumen endobronchial tube size. Anesth Analg 2002;95:501. [PubMed]
- Innes ME. First-attempt success of emergency intubation with bougie was higher than with endotracheal tube plus stylet. Ann Intern Med 2018;169:JC40. [Crossref] [PubMed]
- Xue FS, Sun C, Liu GP. Assessing influence of thermal softened double-lumen endobronchial tube on postoperative airway injury and morbidity: a call for methodology clarification. Br J Anaesth 2017;118:139-40. [Crossref] [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Liang H, Liu J, Wu S, et al. Nonintubated Spontaneous Ventilation Offers Better Short-term Outcome for Mediastinal Tumor Surgery. Ann Thorac Surg 2019;108:1045-51. [Crossref] [PubMed]
- Kiss G, Castillo M. Nonintubated anesthesia in thoracic surgery: general issues. Ann Transl Med 2015;3:110. [PubMed]
- Irons JF, Miles LF, Joshi KR, et al. Intubated Versus Nonintubated General Anesthesia for Video-Assisted Thoracoscopic Surgery-A Case-Control Study. J Cardiothorac Vasc Anesth 2017;31:411-7. [Crossref] [PubMed]
- Shi Y, Yu H, Huang L, et al. Postoperative pulmonary complications and hospital stay after lung resection surgery: A meta-analysis comparing nonintubated and intubated anesthesia. Medicine (Baltimore) 2018;97:e10596 [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Keles S, Kocaturk O. Postoperative discomfort and emergence delirium in children undergoing dental rehabilitation under general anesthesia: comparison of nasal tracheal intubation and laryngeal mask airway. J Pain Res 2018;11:103-10. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-805. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Cai K, Wang X, Ye J, et al. Laryngeal mask anesthesia in video-assisted thoracoscopic surgery for pulmonary bulla: comparison with intubation anesthesia. Nan Fang Yi Ke Da Xue Xue Bao 2013;33:756-60. [PubMed]
- Chen J, Du Q, Lin M, et al. Transareolar Single-Port Needlescopic Thoracic Sympathectomy Under Intravenous Anesthesia Without Intubation: A Randomized Controlled Trial. J Laparoendosc Adv Surg Tech A 2016;26:958-64. [Crossref] [PubMed]
- Hwang J, Shin JS, Son JH, et al. Non-intubated thoracoscopic bullectomy under sedation is safe and comfortable in the perioperative period. J Thorac Dis 2018;10:1703-10. [Crossref] [PubMed]
- Kocatürk C, Kutluk AC, Usluer O, et al. Comparison of awake and intubated video-assisted thoracoscopic surgery in the diagnosis of pleural diseases: A prospective multicenter randomized trial. Turk Gogus Kalp Damar Cerrahisi Derg 2019;27:550-6. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Mao S, Du X, Ma J, et al. A comparison between laryngeal mask airway and endotracheal intubation for anaesthesia in adult patients undergoing NUSS procedure. J Thorac Dis 2018;10:3216-24. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Pompeo E, Dauri M. Is there any benefit in using awake anesthesia with thoracic epidural in thoracoscopic talc pleurodesis? J Thorac Cardiovasc Surg 2013;146:495-7.e1. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Wang S, Zhang J, Cheng H, et al. A clinical evaluation of the ProSeal laryngeal mask airway with a Coopdech bronchial blocker for one-lung ventilation in adults. J Cardiothorac Vasc Anesth 2014;28:900-3. [Crossref] [PubMed]
- Xiang X, Zhou H, Wu Y, et al. Impact of supraglottic device with assist ventilation under general anesthesia combined with nerve block in uniportal video-assisted thoracoscopic surgery. Medicine (Baltimore) 2020;99:e19240 [Crossref] [PubMed]
- Tsai TM, Lin MW, Hsu HH, et al. Nonintubated uniportal thoracoscopic wedge resection for early lung cancer. J Vis Surg 2017;3:155. [Crossref] [PubMed]
- Jiang L, Depypere L, Rocco G, et al. Spontaneous ventilation thoracoscopic thymectomy without muscle relaxant for myasthenia gravis: Comparison with “standard” thoracoscopic thymectomy. J Thorac Cardiovasc Surg 2018;155:1882-9.e3. [Crossref] [PubMed]
- Wang ML, Hung MH, Hsu HH, et al. Non-intubated thoracoscopic surgery for lung cancer in patients with impaired pulmonary function. Ann Transl Med 2019;7:40. [Crossref] [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Hung WT, Hung MH, Wang ML, et al. Nonintubated Thoracoscopic Surgery for Lung Tumor: Seven Years' Experience With 1,025 Patients. Ann Thorac Surg 2019;107:1607-12. [Crossref] [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Thomas DV. Hoarseness and sore throat after tracheal intubation. Small tubes prevent. Anaesthesia 1993;48:355-6. [Crossref] [PubMed]
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg 2010;111:120-8. [Crossref] [PubMed]
- Knoll H, Ziegeler S, Schreiber JU, et al. Airway injuries after one-lung ventilation: a comparison between double-lumen tube and endobronchial blocker: a randomized, prospective, controlled trial. Anesthesiology 2006;105:471-7. [Crossref] [PubMed]
- Puri GD, Hegde HV, Jayant A, et al. Haemodynamic and Bispectral index response to insertion of the Streamlined Liner of the Pharynx Airway (SLIPA): comparison with the laryngeal mask airway. Anaesth Intensive Care 2008;36:404-10. [Crossref] [PubMed]
- Yi MS, Kang H, Kim MK, et al. Relationship between the incidence and risk factors of postoperative nausea and vomiting in patients with intravenous patient-controlled analgesia. Asian J Surg 2018;41:301-6. [Crossref] [PubMed]
- Wen Y, Liang H, Qiu G, et al. Non-intubated spontaneous ventilation in video-assisted thoracoscopic surgery: a meta-analysis. Eur J Cardiothorac Surg 2020;57:428-37. [PubMed]
- Yu MG, Jing R, Mo YJ, et al. Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0224737 [Crossref] [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
(English Language Editors: J. Teoh and J. Chapnick)