Circulating tumor cells in non-small cell lung carcinoma
Abstract Circulating tumor cells (CTCs) are associated with survival of cancer patients. Several methods have been developed to detect circulating tumor cells. The number of CTCs in NSCLC is lower than in other solid tumors. To date, trials are ongoing for a better understanding of CTCs. Besides association with prognosis, CTCs can be used to assess the efficacy of treatment and they are important substrates for molecular profiling of the tumor.
Key words: CTC; circulating tumor cells; HD-CTC; CellSearch; ISET; NSCLC
Lung Cancer is the deadliest cancer worldwide. Annually, in the USA alone over 225,000 people are diagnosed with lung cancer and approximately 160,000 are estimated to die from it in 2012 (1). Decisions in lung cancer treatment are based on imaging rather than on fluid based biomarkers. Unlike CEA in colorectal carcinoma and PSA in prostate cancer, tumor markers are not widely accepted in making treatment decisions for lung cancer patients. Recently, the potential value of circulating tumor cells (CTCs) was shown. CTC based analysis has been envisioned as a “fluid phase biopsy” that enables us to study both quantitative and qualitative properties of the malignancy (2).
The biology of circulating tumor cells, also referred to as circulating epithelial cells, is not well understood. Circulating epithelial cells represent disease derived cells including both cells en route to metastasize and end-stage cells. In fact, in blood the whole spectrum of circulating epithelial cells can be shown (3). CTCs are rare cells among the billions of normal blood cells. At least 13 other methods have been described to identify them by differences in biological and physical properties (4). These methods differ with regard to their sensitivity. To date, the FDA has only approved narrow utility of the CellSearch method for enumerating CTCs in metastasized breast, colorectal and prostate carcinoma in disease prognosis (5,6). In short, the CellSearch method makes use of immunomagnetically enriched epithelial cells. For all the approved utilities, CellSearch method was able to divide patients into long and short term survivors based on the number of circulating tumor cells (7).
Only a few reports have been published on CTCs in NSCLC (2,8-11). When comparing data with the same technology, incidence of CTCs in NSCLC was lower compared with other tumors as reported for the CellSearch method (5), the CTC-chip (12) and as shown in Table 1 (which is derived by combining data from Marrinucci et al. and Wendel et al.) (2,13). Aggregates of CTCs were identified in 50% of NSCLC patients using the same approach (14). Krebs et al. compared CellSearch and ISET (Isolation by Size of Epithelial Tumor Cell) in 40 patients with stage III-IV NSCLC. Samples were more frequently positive for CTCs with the ISET method (80%) compared to CellSearch (23%). Clusters of at least 3 CTCs were detected in 38% of the samples by ISET and in none by CellSearch. No survival analyses were performed (15).
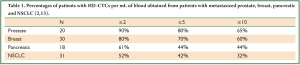
Full Table
The value of CTCs assays in lung cancer diagnostics has yet to be established. CTCs can have prognostic value, even if the diagnostic yield is low. However, for qualitative assessment of tumor properties a test with a high yield is warranted. Sensitivity of some detection methods is too low for this purpose.
Prognostication of disease
In 2011, Krebs et al. reported data from a study using the CellSearch method. Twenty-one out of 101 stage III-IV patients had at least 2 CTCs detected before start of treatment. CTCs before and during treatment were correlated with overall survival and stage of disease (11). Hofman et al. reported a study using ISET. All included patients underwent surgery for NSCLC and all stages were included. Thirty-six percent of all samples were considered to be positive for CTCs. Patients with high level (≥50 cells, 31% of all samples) of suspicious cells turned out to have a worse prognosis (10).
CTCs and response assessment
Changes of CTC numbers during treatment for lung cancer have not been studied widely. Using the CTC-chip, patients with an increase in numbers of circulating tumor cells during treatment had radiographic tumor progression and a reduction of numbers was related to radiographic response (16). CTC numbers (detected by CellSearch) were compared with FDG-PET for to bone metastasized breast cancer and were in agreement up to 80% of cases (17). In patients with stage IV NSCLC, a correlation between changes in number of CTC and FDG-PET or RECIST response was observed. However this was not identified for all time points measured (18). Since FDG-PET for evaluation of (chemo) radiotherapy might vary due to the post irradiation inflammation, CTC detection before, during and after (chemo) radiotherapy might be interesting to compare with FDG-PET response.
Fluid biopsy: qualitative assessment of tumor
Changes in CTCs are thought to play important roles in response assessment. At the present analysis of isolated CTCs makes it possible to identify patients with EGFR, HER2 and KRAS mutations (19). Other markers such as γH2AX, a marker for double strand DNA breaks, were also detected in CTCs (20,21). With a more sensitive CTC detection method, response to for example cisplatin and Tyrosine Kinase Inhibitors can be predicted (8,16).
Conclusions
Highly sensitive methods for use as a fluid biopsy are currently developed. The initial use of these methods is for CTC enumeration as a prognostic tool, which is only of limited use clinically but demonstrates the identification of a relevant rare cell subtype in the blood. The important next step is to use these cells as biopsy material for additional characterization using both traditional immunocytochemistry as well as molecular approaches. NSCLC is particularly attractive due to the clinical need for a real-time fluid biopsy to aid in both the earlier diagnosis and treatment management, and, equally as important, the better understood molecular characteristics of the disease as it relates to possible treatment responses.
Acknowledgements
This manuscript was supported by Award Number U54CA143906 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The HD-CTC assay technology described here has been licensed to Epic Sciences. PK has ownership in Epic Sciences.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 2012;9:016005.
- Nieva J, Wendel M, Luttgen MS, et al. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Phys Biol 2012;9:016004.
- Bednarz-Knoll N, Alix-Panabières C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res 2011;13:228.
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904.
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91.
- Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol 2010;2010:617421.
- Das M, Riess JW, Frankel P, et al. ERCC1 expression in circulating tumor cells (CTCs) using a novel detection platform correlates with progression-free survival (PFS) in patients with metastatic non-small-cell lung cancer (NSCLC) receiving platinum chemotherapy. Lung Cancer 2012;77:421-6.
- Devriese LA, Bosma AJ, van de Heuvel MM, et al. Circulating tumor cell detection in advanced non-small cell lung cancer patients by multi-marker QPCR analysis. Lung Cancer 2012;75:242-7.
- Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35.
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63.
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9.
- Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012;9:016003.
- Cho EH, Wendel M, Luttgen M, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol 2012;9:016001.
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15.
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77.
- De Giorgi U, Mego M, Rohren EM, et al. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J Nucl Med 2010;51:1213-8.
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401.
- Punnoose EA, Atwal SK, Spoerke JM, et al. Molecular biomarker analyses using circulating tumor cells. PLoS One 2010;5:e12517.
- Kummar S, Ji J, Morgan R, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res 2012;18:1726-34.
- Wang LH, Pfister TD, Parchment RE, et al. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res 2010;16:1073-84.


