
Validation of completion lobectomy after wedge resection for ≤20 mm non-small cell lung cancer
Introduction
Anatomical lobectomy with mediastinal lymph node dissection has been the gold standard for surgical treatment of stage I non-small cell lung cancer (NSCLC) for decades (1). Recently, the incidences of small and early-stage lung adenocarcinomas have increased due to improvements in imaging technologies and the widespread use of computed tomography screening (2). A previous study reported that wedge resection as sublobar resection (segmentectomy or wedge resection) may benefit patients with small or early-stage NSCLC (3). In addition, wedge resection plays an important role in the diagnosis and treatment of NSCLC patients with high-risk factors such as limited cardiopulmonary functions.
Completion lobectomy was defined as complete removal of the remaining lung lobe tissue after a previous ipsilateral lung operation (4). This surgical procedure was occasionally performed after wedge resection revealed unexpected NSCLC in the final histopathological examination after intraoperative frozen section diagnosis had suggested benign tumor, metastatic lung tumor or less-invasive adenocarcinoma. However, some NSCLC patients after wedge resection are not considered as candidates for complete resection due to higher age, limited lung function or the presence of other malignant disease. Moreover, the significance of resection of the remaining lobe is uncertain in these clinical scenarios. Some physicians have demonstrated a role of completion lobectomy in achieving favorable prognosis compared to wedge resection, but those studies included pure ground glass opacity adenocarcinoma (4,5). Following these findings, the obvious next step is to identify which populations would most benefit from completion lobectomy for small-size NSCLC. The purpose of this study was to define the necessity of complete lobectomy among patients with small (≤20 mm) NSCLC in our single-institute experience. In addition, we tried to clarify those subgroups showing favorable surgical indications for completion lobectomy after initial wedge resection.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-795).
Methods
Patient selection
We reviewed the medical records of 112 consecutive patients who underwent wedge resection for NSCLC between January 2006 and April 2016 in the Department of Thoracic Surgery at Iwate Medical University. Of these, 68 patients were excluded based on a diameter >20 mm (n=16), pM1 (n=14), simultaneous multiple lung cancers (n=8), clinical and/or pathological node positivity including from diagnostic biopsy (n=3), and Noguchi’s classification A (n=15) or B (n=12). In addition, we excluded patients who were performed curative intentional wedge resection with consolidation tumor ratio ≤0.5 on computed tomography (n=4) as non-invasive NSCLC (6). Finally, a total of 40 patients were eligible. All of 40 patients were not obtained definitive preoperative diagnosis of NSCLC. We recommend completion lobectomy to the patients underwent initial wedge resection and diagnosed unexpected invasive NSCLC in principle. However, the final decision to perform complete resection was made by case-by-case basis according to other malignant disease or patient’s wishes. Baseline variables collected include age, sex, Brinkmann index, forced expiratory volume within 1 s (FEV1), comorbidity, length of postoperative stay, postoperative complications and pathological findings. Postoperative complications within 30 days after surgery were defined as those complications of Clavien-Dindo classification grade II or higher (7). Postoperative pulmonary complications included pneumonia, prolonged air leakage, interstitial pneumonitis, atelectasis, bronchopleural fistula, bronchial asthma, hypoxemia and acute respiratory distress syndrome (8). Cases showing multiple complications were categorized according to the complication that most affected the postoperative outcome. Prolonged air leakage was defined as air leakage lasting ≥7 days (9). This study was approved by the institutional review board (approval No. MH2021-024) and informed consent was taken from all the patients. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Pre- and postoperative staging
Clinical staging was based on chest X-ray, contrast-enhanced computed tomography (CT) of the chest and abdomen, brain CT or magnetic resonance imaging (MRI) and positron emission tomography (PET). All cases underwent the above examinations and evaluation of clinical staging. Postoperative pathological staging was based on the eighth edition of the American Joint Commission on Cancer TNM staging system for NSCLC (10). Histological classification was determined according to the World Health Organization criteria and Noguchi’s classification (11,12).
Surgical procedures
Pulmonary resection was performed under general anesthesia with a double-lumen endotracheal tube for single-lung ventilation. The affected lung was deflated as soon as the pleural space was opened, and deflation was maintained throughout most of the operative course. The patient was placed in the lateral decubitus position. The open method was performed via posterolateral thoracotomy (length, 6–12 cm), dividing portions of the latissimus dorsi and serratus anterior muscles. Complete video-assisted thoracic surgery was performed via 3 ports under monitor vision only. Complete and systematic dissection of hilar and mediastinal lymph nodes was performed in all of complete lobectomy cases. On the other hand, wedge resection was not included lymph node dissection in our institute. After completing the procedure, a sealing test was performed before wound closure and was confirmed during reinflation of the affected lung. A chest tube (Blake®, 19-Fr; Ethicon, Somerville, NJ, USA) was placed from the 5th intercostal trocar to the apex.
Statistical analysis
JMP version 14.1.0 statistical software (SAS Institute, Cary, NC, USA) was used for all statistical analyses in this study. Categorical variables were compared between groups using Pearson’s chi-square test or the Wilcoxon rank-sum test. Disease-specific survival (DSS) was defined as the time from operation to death from lung cancer, which were analyzed to eliminate selection bias in this small sized retrospective study. In this study, recurrence in the stump was defined as local recurrence. On the other hand, metastasis in ipsilateral hilar or mediastinal lymph nodes was define as regional recurrence. Distant recurrence was defined as recurrence in all other sites. Relapse-free survival (RFS) was defined as the time from operation to recurrence or death from any cause. Overall survival (OS) was defined as the time from operation to death from any cause. These survival times were estimated using Kaplan-Meier methods and compared by log rank test. Cox proportional hazard regression modeling was used to analyze prognostic factors and estimate the hazard ratios (HRs) of clinical variables. Differences between groups were considered significant for values of P<0.05. Continuous data are expressed as mean ± standard deviation, and categorical data are expressed as count and proportion.
Results
Patient characteristics
Clinical characteristics of the following 40 patients with NSCLC are summarized in Table 1. In 40 patients with ≤20 mm NSCLC, 23 patients (57.5%) underwent wedge resection alone and 17 patients (42.5%) underwent completion lobectomy after initial wedge resection. All procedures were performed by video-assisted thoracoscopic surgery (VATS) in patients with initial wedge resection. On the other hand, 15 of 17 (88.2%) patients with completion lobectomy group were operated by VATS. The Median length time between complete lobectomy and initial wedge resection was 3.0 months. In final pathological examination, all of 40 cases were diagnosed with negative margin in initial wedge resection. No metastatic lymph nodes were seen in any patients who underwent completion lobectomy. No significant difference between groups was seen in age, sex, Brinkmann index, comorbidity or tumor size. Mean FEV1 was 1.98 L in the wedge resection group and 2.59 L in the completion lobectomy group (P=0.008). Length of postoperative stay was shorter in the wedge resection group (5.1 days) than in the completion lobectomy (10.2 days, P<0.001). Patients in the wedge resection group were more likely to have squamous cell carcinoma (39.1% vs. 4.3%, P=0.04). Conversely, patients in the completion lobectomy group were more likely to have other subtypes of lung cancer (4.3% vs. 23.5%; P=0.04). In the wedge resection group, 1 patient (4.3%) was diagnosed with large cell neuroendocrine carcinoma (LCNEC). In the completion lobectomy group, 2 patients (11.8%) were diagnosed with LCNEC, 1 patient (5.9%) with typical carcinoid and 1 patient (5.9%) with salivary gland-type carcinoma. There were no cases diagnosed with solid or micropapillary subtypes in all of 26 adenocarcinoma cases.
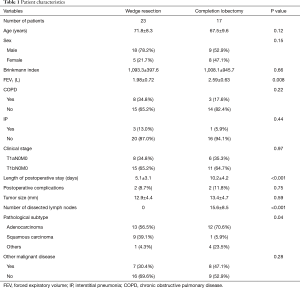
Full table
Survival rate and multivariate analysis for OS and RFS
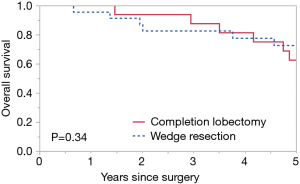
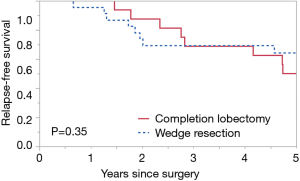
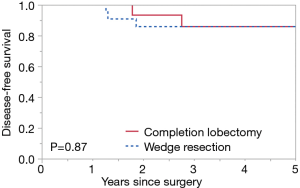
Median follow-up for all patients was 70.4 months, 11 patients had recurrence (Table 2). The 5-year OS rate was 67.9%, 5-year RFS rate was 57.5%, 5-year DSS rate was 86.0%. No significant difference in 5-year OS was seen between the completion lobectomy group (62.5%) and the wedge resection group (72.6%, P=0.34) (Figure 1) however a tendency to better survival was seen in the completion lobectomy group at the early survival curve. Similarly, no significant difference in 5-year RFS was seen between groups (50.0% vs. 64.2%; P=0.35) (Figure 2) and in 5-year DSS (86.2% vs. 86.1%; P=0.87) (Figure 3).

Full table
Multivariate analysis was performed to assess prognostic factors for OS and RFS (Table 3). Clinical factors such as age, sex, tumor size, surgical procedure, pathological subtype and presence of other malignant disease were analyzed. Older age (HR 8.47, 95% CI, 1.44–168.24; P=0.013), male (HR 4.94, 95% CI, 1.31–32.27; P=0.02) were independent prognostic factors associated with poor OS and RFS in this multivariate model.
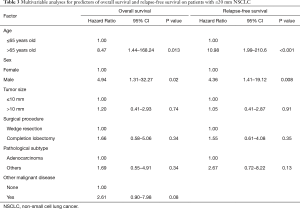
Full table
Discussion
This retrospective study found no significant difference in 5-year OS or RFS between the completion lobectomy group and wedge resection group among patients with ≤20 mm NSCLC. On the other hand, we demonstrated that age (>65 years) was an independent prognostic factor for both OS and RFS according to the results of multivariable analysis. These findings suggest that patients with NSCLC ≤20 mm who undergo completion lobectomy after initial wedge resection may show survival outcomes equivalent to those in patients who undergo wedge resection only.
Several previous reports examining favorable perioperative outcomes have assessed completion lobectomy after wedge resection or radical segmentectomy for NSCLC (13-15). With regard to the prognosis of completion lobectomy, Wang et al. demonstrated completion lobectomy after wedge resection as an appropriate remediation for ≤30 mm adenocarcinoma (4). Although anatomical lobectomy and mediastinal lymph node dissection are recommended as standard surgical procedures for NSCLC, some physicians have described sublobar resection as potentially offering equivalent survival outcomes in selected patients such as those with small-size NSCLC or lepidic-predominant adenocarcinoma (16-18). However, finding from those previous studies were assessed by comparing wedge resection and initial lobectomy group in patients with NSCLC. Moreover, those studies often did not exclude patients with non-invasive adenocarcinoma. As a result, no definitive consensus has been reached on whether to resect the remaining lobe after initial wedge resection in patients with NSCLC diagnosed as invasive carcinoma. In this study, completion lobectomy did not improve OS or RFS compared with wedge resection in patients with ≤20 mm NSCLC. We found that older age and male sex were independent prognostic factors related to worse survival rate. Such results imply that resection of the remaining lobe may not always improve survival outcomes and that other clinical variables are also associated with OS and RFS in patients with small-size NSCLC. These findings may help physicians to consider completion lobectomy after initial wedge resection when final pathology revealed unexpected NSCLC, especially non-lepidic predominant adenocarcinoma.
At the time of writing, the Japan Clinical Oncology Group and West Japan Oncology Group are conducting randomized trials on lobectomy versus segmentectomy for ≤20 mm invasive lung cancers (JCOG0802/WJOG4607L) (6). Initial results from that randomized trial showed the noninferiority of perioperative outcomes in segmentectomy compared with lobectomy (19). Our study mainly used pathological classification as an exclusion criterion for non-invasive adenocarcinoma (12). Patients with pathologically confirmed non-invasive adenocarcinoma show excellent prognosis (5-year OS rate 95.7%) even after wedge resection (20,21). Therefore, some degree of consensus has been reached regarding sublobar resection for small non-invasive lung cancer. Moreover, segmentectomy might become a standard surgical procedure after the JCOG0802/WJOG4907L trials have been completed. Further multicenter studies focusing on wedge resection versus lobectomy should be conducted for invasive lung cancer excluding non-invasive adenocarcinoma.
For patients who undergo sublobar resection for NSCLC, appropriate lymph node assessment is essential for operations with curative intent, because wedge resection does not usually include lymph node dissection. A previous study identified metastatic lymph nodes in 4.3% of patients considered eligible for wedge resection (22). In this study, we evaluated preoperative nodal staging using CT and PET findings and excluded three of 112 patients (2.7%) diagnosed as pathological node positive by lymph node sampling. Consequently, no metastatic lymph nodes were identified in any patients who underwent completion lobectomy. A previous study of wedge resection for ≤20 mm NSCLC reported that lymph node dissection status was not a prognostic factor (23,24). Furthermore, Moon et al. demonstrated that lymph node upstaging did not occur in clinical N0 NSCLC <1.3 cm in diameter with a consolidation tumor ratio <0.6 (25,26). Such findings together with the present study provide support for the possibility that completion lobectomy may be unnecessity for patients with small invasive lung cancer diagnosed as clinical N0. However, surgeons should make the final determination of the surgical procedure and extent of lymph node dissection on the basis of clinical diagnosis.
This study showed several limitations that warrant consideration. First, the population in this study included some degree of selection bias. In particular, we could not assess pathological nodal staging in patients from the wedge resection group, as they did not undergo lymph node sampling. On the other hand, more patients with adenocarcinoma were present in the completion lobectomy group than in the wedge resection group. Some researchers consider that significantly more lung cancer-specific deaths occur among patients with adenocarcinoma than among patients with squamous cell carcinoma (27). Such selection bias could have contributed to the worsened RFS and OS in the completion lobectomy group compared to wedge resection group. Second, all of 40 patients were not diagnosed NSCLC before the initial operation, which might lead to require the completion lobectomy according to misdiagnosis of frozen section. Liu et al. reported the concordance rate was 84.4% between frozen section and final pathology in ≤30 mm adenocarcinoma (28). Therefore, these misdiagnoses potentially cause a bias and preoperative tissue diagnosis is preferred for correct clinical decision to perform lobectomy. Third, this study was retrospective nature and included only a small number of patients from a single institute. Therefore, the findings may not be generalizable to other institutions and a large, multicenter study is necessary to confirm the present findings.
In conclusion, completion lobectomy after wedge resection did not impact OS or RFS compared with wedge resection among patients with ≤20 mm NSCLC in this study. These findings suggested that wedge resection alone is reasonable in selected patients with ≤20 mm NSCLC unexpectedly diagnosed NSCLC in the final pathological examination.
Acknowledgments
The authors wish to thank Tatsuo Tanita for his variable suggestion.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-795
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-795
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-795
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-795). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by institutional board (approval No. MH2021-024) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Moon Y, Sung SW, Moon SW, et al. Risk factors for recurrence after sublobar resec-tion in patients with small (2 cm or less) non-small cell lung cancer presenting as a solid-predominant tumor on chest computed tomography. J Thorac Dis 2016;8:2018-26. [Crossref] [PubMed]
- Wang Y, Wang R, Zheng D, et al. The indication of completion lobectomy for lung adenocarcinoma ≤3 cm after wedge resection during surgical operation. J Cancer Res Clin Oncol 2017;143:2095-104. [Crossref] [PubMed]
- Ohtaka K, Hida Y, Kaga K, et al. Limited resection and two-staged lobectomy for non-small cell lung cancer with ground-glass opacity. J Cardiothorac Surg 2013;8:111. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth 2017;118:317-34. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of pro-longed air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. Union for International Cancer Con-trol (UICC) TNM Classfication of Malignant Tumors. 8th edition. New York: Wiley-Blackwell, 2017.
- Travis WD, Brambilla E, Nicholson AG, et al. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer, 2015.
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Holbek BL, Petersen RH, Hansen HJ. Is it safe to perform completion lobectomy after diagnostic wedge resection using video-assisted thoracoscopic surgery? Gen Thorac Cardiovasc Surg 2016;64:203-8. [Crossref] [PubMed]
- Nomori H, Mori T, Izumi Y, et al. Is completion lobectomy merited for unanticipated nodal metastases after radical segmentectomy for cT1 N0 M0/pN1-2 non-small cell lung cancer? J Thorac Cardiovasc Surg 2012;143:820-4. [Crossref] [PubMed]
- Takahashi Y, Miyajima M, Tada M, et al. Outcomes of completion lobectomy long after segmentectomy. J Cardiothorac Surg 2019;14:116. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Zhang Z, Feng H, Zhao H, et al. Sublobar resection is associated with better periop-erative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis 2019;11:1838-48. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [Crossref] [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [Crossref] [PubMed]
- Stiles BM, Kamel MK, Nasar A, et al. The importance of lymph node dissection ac-companying wedge resection for clinical stage IA lung cancer. Eur J Cardiothorac Surg 2017;51:511-7. [PubMed]
- Mimae T, Miyata Y, Tsutani Y, et al. Wedge resection as an alternative treatment for octogenarian and older patients with early-stage non-small-cell lung cancer. Jpn J Clin Oncol 2020;50:1051-7. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Prognosis after wedge resection in patients with 8th edition TNM Stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis 2019;11:2361-72.
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocar-cinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Kawase A, Yoshida J, Ishii G, et al. Differences between squamous cell carcinoma and adenocarcinoma of the lung: are adenocarcinoma and squamous cell carcinoma prognostically equal? Jpn J Clin Oncol 2012;42:189-95. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adeno-carcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]


