
Modified “in situ” arch replacement with an integrative frozen elephant trunk device for acute type A aortic dissection
Introduction
Total arch replacement (TAR) and frozen elephant trunk (FET) is a widely used arch repair method for acute type A aortic dissection (aTAAD), which is beneficial for both perioperative distal organ perfusion and long-term aortic reshape effects (1-4). However, the method used for TAR with separate reimplantation of the supra-aortic branches (TAR) is a complex technique with severe trauma, increasing the risk of neurologic events postoperatively (5). Intimal tears located in the supra-aortic branches and/or large curve of the aortic arch, aneurysm enlargement of the aortic arch and Marfan syndrome are risk factors for late dilation and reintervention, which could be avoided by applying TAR with separate reimplantation (6,7). However, for patients without these indicators, the necessity for TAR with separate reimplantation is controversial. An alternative approach for TAR is re-anastomosing the supra-aortic branches “en bloc”, or the so-called “island”. With the advances of FET, limited experiences combining “island” TAR with FET have been introduced previously. Thus, we introduced a modified island total arch replacement (MiTAR) combined with FET for aTAAD and compared intraoperative and postoperative outcomes with separate reimplantation TAR methods.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-75).
Methods
Patients
A total of 507 patients with aTAAD underwent surgery at Nanjing Drum Tower Hospital between January 2018 and December 2019. Among them, several methods for aortic arch management were applied according to the indications and the surgeons’ selection. A newly introduced method named MiTAR was used in our centre and is reported in this article for the first time. A total of 57 patients received MiTAR, while 138 patients received TAR with separate reimplantation of the supra-aortic branches (TAR), which is the standard approach for extent arch replacement in China and was introduced previously (3). We measured the diameter at the aortic arch level, pulmonary artery bifurcation level, diaphragm level and renal artery level preoperatively (T0), postoperatively (T1) and at the follow-up (T2).
We retrieved the data retrospectively by a review of hospital records, and individual consent for this retrospective analysis was waived. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The current study was approved by the institutional review board of Nanjing Drum Tower Hospital (2020-185-01).
Surgical procedures
MiTAR is a simplified “island” TAR with a FET. We chose aTAAD patients with the following indications to receive MiTAR or TAR: aortic dissection involving the entire aortic arch and descending aorta. However, patients with Marfan syndrome, primary intimal tears (PITs) located in the large curve of the aortic arch and/or supra-arch vessels and a dilated aortic arch (≥45 mm) are not suitable for MiTAR and must undergo TAR. The surgical procedure for MiTAR is applied during the deep hypothermic circulation arrest period. After circulation arrest and cerebral perfusion initiation, the aortic arch is resected along the small curve, and the entire “island” aortic arch is left connected with the proximal thoracic descending aorta in situ. A suitable sized FET device (Microport, Shanghai, China) is used and inserted into the descending aorta at zone 3 as the proximal landing zone. The proximal part of the FET device is a length of 2 cm Dacron vessel, which is cut into an empty zone in the site of supra-arch vessels after insertion. The remaining proximal prosthetic vessel was then continuously anastomosed endovascularly with a native aortic vessel with a two-sided 4-0 polypropylene stitch. The stitch begins from the bottom of the arch and goes forward to the top of the arch both anteriorly and posteriorly. Then, a straight Dacron vessel is cut into a sloped shape and continuously anastomosed with the remaining “island arch” using 4-0 polypropylene (Figure 1). After replacing the aortic arch, the extracorporeal circulation is resumed, and warming is started. Then, we repair the proximal aortic root.
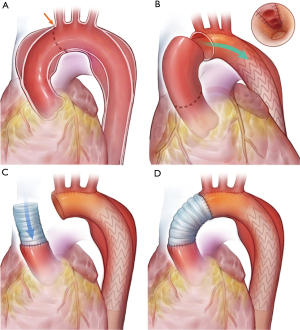
The TAR procedure with branched arch replacement and FET is performed as reported widely in previous articles.
Statistical analysis
All data are presented as n (%) for categorical variables and mean ± standard deviation for continuous variables. Between-group differences were analysed using Student’s t-test or the Mann-Whitney U-test for continuous variables and a chi-square or Fisher’s exact test for categorical variables. Data analysis was performed using SPSS 23 (IBM, Herrenberg, Germany). A P value of less than 0.05 was considered statistically significant.
Results
Preoperative characteristics
Patients receiving MiTAR were older than those receiving TAR (52.1±12.7 vs. 48.9±11.3 years; P=0.078). Male patients accounted for the majority. A total of 78.9% of all MiTAR patients and 77.5% of TAR patients (P=0.829) were diagnosed with hypertension. Three patients in the TAR group had Marfan syndrome. A total of 5.3% of the MiTAR patients and 3.6% of the TAR patients had diabetes (P=0.600). A total of 10.5% MiTAR and 8.7% TAR patients had hypotension upon presentation, and the rates of concomitant pericardial tamponade were 5.3% and 9.4% (P=0.337), respectively. The proportions of cerebral, limb, mesenteric and myocardial malperfusion before the operation were not significantly different between the two groups.
A total of 38.6% of MiTAR patients and 42.0% of TAR patients had PITs located in the ascending aorta. No case with PITs was located in the large curve of the arch and descending aorta in MiTAR, while the rate was 10.9% and 4.3% in TAR. A total of 61.4% in MiTAR and 42.8% in TAR had PIT in the small curve of the arch.
Operation data and outcomes
The times of cardiopulmonary bypass (CPB), clamp and HCA were significantly shorter in the MiTAR group (209.3 vs. 267.1, P=0.000; 147.9 vs. 190.0, P=0.000; 34.0 vs. 39.4, P=0.003, respectively). Most patients received femoral and axillary double arterial cannulation, and the proportion of axillary arterial cannulation in the MiTAR group was higher than that in the TAR group (19.3% vs. 7.2%; P=0.014). The root procedures, including root reconstruction and the Bentall procedure, were similar, and concomitant coronary artery bypass graft (CABG) was also similar. The mean volume of the intraoperative transfusion was significantly lower in the MiTAR group, which was 5.9 units of red blood cells (RBCs) (vs. 8.5 units, P=0.000), 758.3 mL of fresh frozen plasma (FFP) (vs. 930.4 mL, P=0.000), 12.5 units of platelets (vs. 17.5 units, P=0.000) and 9.4 units of cryoprecipitate (vs. 16.6 units, P=0.000).
The volume of drainage over 24 hours was 553.2 mL in MiTAR and 874.8 mL in TAR (P=0.000). The duration of mechanical ventilation was shorter in the MiTAR group (37.6±45.1 vs. 59.4±102.1 hours; P=0.141). No differences were found in the rates of postoperative complications (Table 1).
Table 1
| Variables | Whole | ||
|---|---|---|---|
| MiTAR (N=57) | TAR (N=138) | P value | |
| Age (years), mean ± SD | 52.1±12.7 | 48.9±11.3 | 0.078 |
| Gender-male, n (%) | 42 (73.7) | 111 (80.4) | 0.298 |
| BMI (kg/m2), mean ± SD | 26.8±4.0 | 25.9±3.9 | 0.145 |
| Hypertension, n (%) | 45 (78.9) | 107 (77.5) | 0.829 |
| Marfan syndrome, n (%) | 0 (0) | 3 (2.2) | 0.263 |
| DM, n (%) | 3 (5.3) | 5 (3.6) | 0.600 |
| Hypotension, n (%) | 6 (10.5) | 12 (8.7) | 0.689 |
| Tamponade, n (%) | 3 (5.3) | 13 (9.4) | 0.337 |
| Malperfusion, n (%) | |||
| Cerebral ischemia | 3 (5.3) | 6 (4.3) | 0.782 |
| Limb ischemia | 10 (17.5) | 18 (13.0) | 0.416 |
| Mesenteric ischemia | 1 (1.8) | 0 (0) | 0.586 |
| Myocardial ischemia | 2 (3.5) | 2 (1.4) | 0.357 |
| PIT location, n (%) | 0.974 | ||
| Ascending aorta | 22 (38.6) | 58 (42.0) | |
| Aortic arch-large curve | 0 (0) | 15 (10.9) | |
| Aortic arch-small curve | 35 (61.4) | 59 (42.8) | |
| Descending aorta | 0 (0) | 6 (4.3) | |
| CPB (min), mean ± SD | 209.3±71.7 | 267.1±67.6 | 0.000 |
| Clamp (min), mean ± SD | 147.9±43.3 | 190.0±46.0 | 0.000 |
| HCA (min), mean ± SD | 34.0±9.1 | 39.4±11.6 | 0.003 |
| Arterial cannulation, n (%) | |||
| Ascending aorta | 0 (0) | 1 (0.7) | 0.520 |
| Femoral artery | 12 (21.1) | 19 (13.8) | 0.207 |
| Axillary artery | 11 (19.3) | 10 (7.2) | 0.014 |
| Femoral + axillary artery | 34 (59.6) | 108 (78.3) | 0.008 |
| Root procedure, n (%) | 0.288 | ||
| Root reconstruction | 41 (71.9) | 108 (78.3) | |
| Bentall procedure | 11 (19.3) | 21 (15.2) | |
| Concomitant CABG, n (%) | 4 (7.1) | 12 (8.7) | 0.681 |
| Transfusion intraoperative, mean ± SD | |||
| RBC (units) | 5.9±2.5 | 8.5±2.7 | 0.000 |
| FFP (mL) | 758.3±243.2 | 930.4±211.9 | 0.000 |
| Platelets (units) | 12.5±5.4 | 17.5±4.8 | 0.000 |
| Cryoprecipitate (units) | 9.4±3.1 | 16.6±5.4 | 0.000 |
| Drainage during 24 hours (mL), mean ± SD | 553.2±370.5 | 874.8±260.9 | 0.000 |
| Mechanical ventilation (hours), mean ± SD | 37.6±45.1 | 59.4±102.1 | 0.141 |
| Re-intubation, n (%) | 3 (5.3) | 12 (8.7) | 0.414 |
| ICH, n (%) | 0 (0) | 3 (2.2) | 0.263 |
| Stroke, n (%) | 5 (8.8) | 9 (6.5) | 0.581 |
| Paraplegia, n (%) | 1 (1.8) | 4 (2.9) | 0.647 |
| ARF, n (%) | 19 (33.3) | 57 (41.3) | 0.300 |
| CRRT, n (%) | 7 (12.3) | 20 (14.5) | 0.685 |
| Re-exploration, n (%) | 0 (0) | 5 (3.6) | 0.146 |
| Hospital (days), mean ± SD | 18.4±11.5 | 19.6±10.0 | 0.492 |
| ICU (days), mean ± SD | 7.7±11.7 | 6.4±6.9 | 0.437 |
| 30-day mortality, n (%) | 4 (7.0) | 16 (11.6) | 0.339 |
MiTAR, modified island total arch replacement; TAR, total arch replacement; BMI, body mass index; DM, diabetic mellitus; CPB, cardiopulmonary bypass; HCA, hypothermic circulation arrest; CABG, coronary artery bypass graft; RBC, red blood cell; FFP, fresh frozen plasma; ICH, intracranial hemorrhage; ARF, acute renal failure; CRRT, continuous renal replacement therapy; ICU, intensive care unit; SD, standard deviation.
Follow-up results
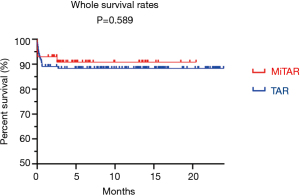
During a median follow-up period of 10.7 months, the overall survival rate was 91.2% in MiTAR and 88.4% in TAR (P=0.589). Four and 16 patients in each group died after surgery before discharge. One patient in the MiTAR group died 2.5 months after discharge due to pulmonary failure, while no patient died after discharge in the TAR group (Figure 2).
At admission before the operation, the size of the aorta from the four levels based on computed tomographic angiography (CTA) was similar between the two groups (Table 2). At the aortic arch level, the diameter in MiTAR was smaller than that in TAR, and the differences were meaningful during the follow-up stage after the operation (30.0 vs. 31.0 mm, P=0.043). At the pulmonary artery bifurcation level, the size of the descending aorta was smaller in MiTAR (29.0 vs. 31.0 mm, P=0.043). The size of the abdominal aorta at both the level of the diaphragm and the renal artery were all smaller in MiTAR after surgery, but no significant difference existed.
Table 2
| Level of aorta | Group | T0 | T1 | T2 |
|---|---|---|---|---|
| Aortic arch level (mm) | MiTAR | 35.0±3.4 | 29.8±4.4 | 30.0±1.9 |
| TAR | 35.8±4.2 | 30.5±4.8 | 31.0±2.2 | |
| P value | 0.614 | 0.584 | 0.043 | |
| Pulmonary artery bifurcation level (mm) | MiTAR | 34.7±3.1 | 33.8±4.8 | 29.0±1.9 |
| TAR | 33.5±4.6 | 35.2±5.5 | 31.0±2.2 | |
| P value | 0.426 | 0.362 | 0.043 | |
| Diaphragm level (mm) | MiTAR | 31.6±4.2 | 32.3±4.2 | 26.6±13.5 |
| TAR | 29.2±3.9 | 33.1±7.2 | 31.8±5.8 | |
| P value | 0.058 | 0.668 | 0.303 | |
| Renal artery level (mm) | MiTAR | 24.6±4.5 | 23.9±3.4 | 22.1±7.9 |
| TAR | 23.3±4.3 | 25.4±4.0 | 25.4±5.1 | |
| P value | 0.445 | 0.176 | 0.269 |
T0: at admission before operation; T1: early stage after operation; T2: follow-up stage after operation. MiTAR, modified island total arch replacement; TAR, total arch replacement.
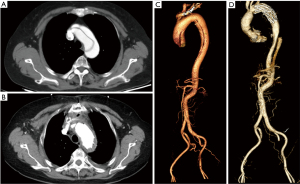
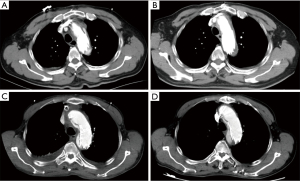
The ideal postoperative aortic morphology should look like Figure 3. Unfortunately, four patients who survived after discharge had aortic arch endoleak (Figure 4). The endoleak blood flow disappeared in two patients during follow-up (Figure 4A,4B for one) and remained in the other two (Figure 4C,4D for one). However, the aortic size of these two patients with persistent endoleak did not change; therefore, we decided to continue the follow-up.
Discussion
In a meta-analysis comparing conservative arch replacement (CAR) and total aortic arch replacement (TAR) for aTAAD, Hsieh et al. found that CAR had a lower early mortality rate [relative risk (RR) =0.77; 95% confidence interval (CI): 0.61–0.96] and a shorter operative time [CPB time, mean difference =−53.09; 95% CI: −56.68 to −49.50; circulatory arrest time, mean difference =−8.09; 95% CI: −9.04 to 7.15; antegrade cerebral perfusion (ACP), mean difference =−28.62; 95% CI: −30.23 to −27.00], while the incidence of early-stage postoperative complications was similar to TAR. Otherwise, the rate of aortic reintervention was lower in the TAR group, but the difference was not significant (5.3% vs. 7.6%, P=0.10) (8). In another previous meta-analysis, Li et al. analysed 8 representative clinical studies and reported that the rate of freedom from reoperation at 5 and 10 years was similar in the TAR and CAR groups, but the rate of complete thrombosis of the false lumen was significantly higher in the TAR group than in the CAR group (P<0.05) (9).
The results from the International Registry of Acute Aortic Dissection (IRAD) reported that the overall in-hospital mortality was 14.2%, with no difference between CAR and TAR (10). Larsen et al. (10) also found that a stent in the descending aorta for aTAAD in the same stage of central repair could open the true lumen of the distal aorta and preserve the perfusion of distal organs. Therefore, an increasing number of surgeons choose extent arch repair with stents in the descending aorta. However, extent arch repair would increase the extent of surgical injury, especially for acute and severe critical ill stage patients with aTAAD (11). In our experience, extent arch repair would not increase the surgical risk for selected cases. For patients with intimal tears located in the aortic arch, extent arch repair is necessary, and a stent in the descending aorta placed during a single staged surgery will open the true lumen and provide an anastomosis end for proximal suture.
The technique of TAR that is currently widely used in China was introduced by Sun et al. (3). Separate replacement of supra-arch branches is necessary for intimal tears located in the supra-arch vessels and/or the large curve of the aortic arch. Patients with intimal tears located in the supra-arch vessels have a higher rate of reoperation and a quicker growth rate of the residual aorta (7,12). For patients with Marfan syndrome or other connective tissue diseases, residual aortic tissue left after “island” arch replacement will eventually re-dilate and result in reintervention (6). Otherwise, both “island” arch replacement and separate branched arch replacement are suitable and effective. A study from the ARCH registry comparing “island” and separate branched arch replacement reported that separate branched arch replacement was not associated with an increased mortality rate or an increased risk of neurologic events [odds ratio (OR) 1.56, 95% CI: 1.06–2.29; P=0.023] (5); among these patients, 33.1% in the “island” group and 21.1% in the separate branched group received FET during the same stage.
Several methods have been introduced for “island” arch replacement combined with FET. Vallabhajosyula et al. ntroduced a method of hemiarch replacement with concomitant antegrade stent grafting of the descending thoracic aorta. The stent was a GoreTAG stent graft, which is applied for endovascular therapy and off-label use in open surgery. Thirty patients with arch tears received this procedure, and the overall mortality was 13% (4/30). The advantages over TAR were lower CPB and circulatory arrest times, a lower rate of mortality, an improved survival rate and a decreased rate of false lumen thrombosis (13). However, the stent device GoreTAG is not designed for antegrade implantation during open surgery. Roselli et al. reported 72 patients who underwent simplified FET and achieved good results. In this group, the first 39 cases were treated by modifying a stent graft, as we introduced in this article. Other patients received stent grafts with fenestration below the left subclavian artery or direct branch vessel stent grafting into the left subclavian artery. The overall mortality rate was 4.2%, and the rates of stroke (4.2%) and spinal injury (4.2%) were all low and acceptable. However, the stent device was not described in detail (14).
Zhu et al. reported a method similar to ours: the proximal part of the stent device of Cronus was modified as an “island” part and anastomosed with the remaining arch, but the left subclavian artery was covered and reconstructed through bypass to the carotid artery (15). When compared with separated branched cases, the “island” group had a significantly longer time of selective antegrade cerebral perfusion (33 vs. 24 min, P=0.001), while the mortality rate was similar (5.6% vs. 5.7%, P=0.981). The authors thought that this approach may be an acceptable alternative technique (16).
Overlooking the specific operation details, the surgeons mentioned above all had the same opinion: dissection involving the aortic arch is beneficial for extent arch replacement combined with FET, but not all patients need to undergo a separate branched supra-arch replacement. An “island” or “en bloc” arch replacement also achieves a good result. However, it should be noted that the implantation of the stent will introduce the risk of an endoleak, especially when the proximal landing zone is still dissection tissue, although the occurrence of endoleaks has not been reported in the above literature. Four of our MiTAR patients had this complication during an early stage; two of these patients were lost to follow-up, and the other two had no significant change in diameter. To avoid endoleak, we need to fix the stent as firmly as possible with the aid of transluminal sutures from inside to outside.
Conclusions
This MiTAR combined with FET is a safe and effective technique according to our experience. This technique facilitates true cavity perfusion and shaping of the distal aorta while simplifying arch manipulation, avoiding excessive trauma and neurological events caused by separated branched supra-arch replacement.
Limitations
Our study was a retrospective analysis at a single center. The number of cases is still small, and the enrolment criteria are strict. The follow-up duration is still limited and cannot reflect the late outcomes of this arch repair surgery.
Acknowledgments
Funding: This work has been supported by the National Natural Science Foundation of China (No. 81970401) and Jiangsu Provincial Key Medical Discipline (ZDXKA2016019).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-75
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-75
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-75
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-75). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We retrieved the data retrospectively by a review of hospital records, and individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The current study was approved by the institutional review board of Nanjing Drum Tower Hospital (2020-185-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shrestha M, Fleissner F, Ius F, et al. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far?†. Eur J Cardiothorac Surg 2015;47:361-6; discussion 366. [Crossref] [PubMed]
- Shrestha M, Haverich A, Martens A. Total aortic arch replacement with the frozen elephant trunk procedure in acute DeBakey type I aortic dissections. Eur J Cardiothorac Surg 2017;51:i29-34. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Ma WG, Zhang W, Wang LF, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg 2016;151:1581-92. [Crossref] [PubMed]
- Schoenhoff FS, Tian DH, Misfeld M, et al. Impact of reimplantation technique of supra-aortic branches in total arch replacement on stroke rate and survival: results from the ARCH registry. Eur J Cardiothorac Surg 2018;54:1045-51. [Crossref] [PubMed]
- Schoenhoff FS, Kadner A, Czerny M, et al. Should aortic arch replacement be performed during initial surgery for aortic root aneurysm in patients with Marfan syndrome? Eur J Cardiothorac Surg 2013;44:346-51; discussion 351. [Crossref] [PubMed]
- Heo W, Song SW, Lee KH, et al. Surgery for acute Type I aortic dissection without resection of supra-aortic entry sites leads to unfavourable aortic remodelling. Eur J Cardiothorac Surg 2018;54:34-41. [Crossref] [PubMed]
- Hsieh WC, Kan CD, Yu HC, et al. Ascending aorta replacement vs. total aortic arch replacement in the treatment of acute type A dissection: a meta-analysis. Eur Rev Med Pharmacol Sci 2019;23:9590-611. [PubMed]
- Li B, Ma WG, Liu YM, et al. Is extended arch replacement justified for acute type A aortic dissection? Interact Cardiovasc Thorac Surg 2015;20:120-6. [Crossref] [PubMed]
- Larsen M, Trimarchi S, Patel HJ, et al. Extended versus limited arch replacement in acute Type A aortic dissection. Eur J Cardiothorac Surg 2017;52:1104-10. [Crossref] [PubMed]
- Lio A, Nicolò F, Bovio E, et al. Total Arch versus Hemiarch Replacement for Type A Acute Aortic Dissection: A Single-Center Experience. Tex Heart Inst J 2016;43:488-95. [Crossref] [PubMed]
- Heo W, Song SW, Lee KH, et al. Residual Arch Tears and Major Adverse Events After Acute DeBakey Type I Aortic Dissection Repair. Ann Thorac Surg 2018;106:1079-86. [Crossref] [PubMed]
- Vallabhajosyula P, Gottret JP, Robb JD, et al. Hemiarch replacement with concomitant antegrade stent grafting of the descending thoracic aorta versus total arch replacement for treatment of acute DeBakey I aortic dissection with arch tear†. Eur J Cardiothorac Surg 2016;49:1256-61; discussion 1261. [Crossref] [PubMed]
- Roselli EE, Idrees JJ, Bakaeen FG, et al. Evolution of Simplified Frozen Elephant Trunk Repair for Acute DeBakey Type I Dissection: Midterm Outcomes. Ann Thorac Surg 2018;105:749-55. [Crossref] [PubMed]
- Zhu JM, Qi RD, Chen L, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Preservation of autologous brachiocephalic vessels. J Thorac Cardiovasc Surg 2015;150:101-5. [Crossref] [PubMed]
- Lin Y, Ma WG, Zheng J, et al. Supra-aortic vessel reconstruction in total arch replacement for acute type A dissection: Comparison of en bloc and separate graft techniques. Asian J Surg 2019;42:482-7. [Crossref] [PubMed]


