Propofol decreases CD8+ T cells and sevoflurane increases regulatory T cells after lung cancer resection: a randomized controlled trial
Introduction
Lung cancer is the leading cause of new cancers and cancer deaths worldwide. In 2018, there were 2.1 million new lung cancers and 1.8 million deaths from lung cancer, accounting for about 20% of all cancer deaths (1). About 30% of Japanese people die of cancer, with lung cancer being the leading cause of death among men, and second leading cause among women.
Of the 42,482 patients with primary lung cancer, 42,107 (99%) underwent surgery in 2016; 5,261 (12.5%) of these patients were over 80 years old, and the population was older (1). Aging causes immunosuppression; age-related immune weakness is thought to be one of the causes of cancer development and poor prognosis (2). With aging, the thymus gland atrophies and impairs various functions of the T and B cells, causing a decline in acquired immune function. In addition, aging increases the expression of programmed death-1 (PD-1), which is one of the suppressive receptors for T cell activation (3) and regulatory T cells and inhibits T cell function (4). Passive and active smoking, chronic obstructive pulmonary disease, several infectious diseases such as tuberculosis, and a history of interstitial pneumonia, in addition to immunosenescence, results in chronic inflammation, leading to a pre-cancer state (5).
Anaesthetics also have an immunosuppressive effect during surgery. Volatile inhalation anaesthesia induces a significantly worse survival rate than propofol in patients after gastrointestinal (6), as well as breast, colon, and rectal cancer surgery (7). In patients who had undergone breast cancer surgery, sevoflurane reduced natural killer (NK) cell-activating receptors; however, propofol did not (8). In patients who had undergone elective minimally invasive partial discectomy, sevoflurane reduced the number of CD3+, CD4+, and CD8+ cells (9). Nevertheless, several retrospective cohort studies have found no significant difference in overall survival with propofol and volatile anaesthetics in patients with lung cancer (10). Although some anaesthetics, such as ketamine, thiopental, halothane, and propofol, reduced NK activity and promoted retention or metastasis of lung cancer in mice (11), differences in immunosuppression by choice of intraoperative anaesthesia in lung cancer patients remains unclear. The purpose of this study was to clarify the relationship between peri-operative immunosuppression and anaesthetic agents in patients undergoing lung cancer surgery, to aid the selection of surgical anaesthesia.
We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-878).
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institution ethics board of Juntendo University Hospital (No. 17-286), Tokyo, Japan (Chairperson Prof. Atsushi Okuzawa) on 31 May 2018, and registered at Clinical Trials Registry System, UMIN-CTR: UMIN000031911. Written informed consent was obtained from all participants.
Participants
Patients who underwent scheduled resection for primary lung cancer at Juntendo University Hospital were enrolled from June 13, 2018 to July 24, 2019. This study was a three-arm parallel study and allocation ratio was closed to 1:1:1. The patients were randomly divided to three groups regarding to general anaesthesia methods [volatile desflurane (group D), volatile sevoflurane (group S), and intravenous propofol (group P)]. On acquiring the written informed consent from the participant on the day before surgery, they were randomized into each group by using permuted block randomization, in sequence of registration. The permuted blocks including 25 for each group had been made by Juntendo Clinical Research and Trial Center in advance immediately after IRB approval. Exclusion criteria included a lack of informed consent, age less than 20 years, immunological disease, pre-treatment with chemoradiotherapy, taking immunosuppressive agents or steroids, and simultaneous bilateral surgery. Primary endpoint is to measure the changes in the number of immune response cells. Peripheral blood mononuclear cells were separated from the blood samples. CD4+ and CD8+ T cells, programmed death 1 (PD-1) on CD4+ and CD8+ T cells, and regulatory T cells were measured by flow cytometry. These parameters were compared with each anesthetic used in the perioperative period. A preliminary study was conducted on 10 patients in each group during the first 3 months from starting the study. Among them, CD4+ ratio in peripheral blood of pre- and post- surgery was calculated, and the required number of cases in this study was calculated by power calculation (significance level 0.05, power: 0.8). More than 20 patients were calculated for each group.
Anaesthesia method
General anaesthesia was performed with desflurane, propofol, or sevoflurane combined with epidural anaesthesia, inserted into the Th5-7 intervertebral space. Before inducing general anaesthesia, the arterial catheter was placed in the radial artery under local anaesthesia, where the first blood sample was collected.
In the desflurane and sevoflurane groups, general anaesthesia was induced with remifentanil (0.3–0.4 µg/kg/min), propofol (1–1.5 mg/kg), and rocuronium (0.6 mg/kg); in the propofol group, general anaesthesia was induced with remifentanil (0.3–0.4 µg/kg/min), propofol (target blood concentration 3.0 µg/mL by target-controlled infusion), and rocuronium (0.6–1 mg/kg).
In all groups, after achieving muscle relaxation, the patients were intubated via the trachea using the left sided Double lumen tube. Remifentanil was controlled in the range of 0.1–0.25 µg/kg/min according to blood pressure and HR. Occasionally, a vasopressor such as phenylephrine (0.1 mg) was used to maintain a range within ±20% of the initial value, while 10 mg Rocuronium was added during each surgery if needed.
At the induction of general anaesthesia, 4 mL of 0.25% levobupivacaine, 50 µg fentanyl, and 1–2 mg morphine was administered via the epidural catheter, followed by continuous epidural infusion of levobupivacaine (3 mL/h) and morphine (2–3 mg/day). The depth of anaesthesia was assessed using bispectral index (BIS) monitoring and was maintained in the range of 40–60 in the three groups. At the end of the surgery, anaesthetic administration was stopped, and immediately after that, the second blood sample was collected. Sugammadex was used to recover from muscle relaxants if needed.
Antibodies
The following human antibodies (Bio-Legend, San Diego, CA, USA) specific to the surface markers were used: anti-CD3-APC/Cy7 (clone HIT3a) for T cells, anti-CD4-PerCP/Cy5.5 (clone SK3) for CD4+ T cells, anti-CD8-PE/Cy7 (clone SK-1) for CD8+ T cells, anti-CD279-APC (clone EH12.2H7) for programmed death-1, anti-CD25-FITC (clone BC96), anti-CD127-PE (clone A019D5); regulatory T cells were identified as CD25+ CD127− in CD4+ T cells.
Sample collection and analysis of immune cells
Both before induction of anaesthesia and at the end of surgery, 8 ml blood samples were collected in a cell preparation tube containing Ficoll and sodium citrate (Vacutainer cell preparation tube; BD Biosciences, Franklin Lakes, NJ, USA). Within 2 h after blood collection, the tubes were centrifuged to separate peripheral blood mononuclear cells (PBMCs) and plasma. PBMCs were stained with the surface markers previously described and quantified via flow cytometry. As a control, PBMCs were also incubated with the appropriate isotype control antibodies. Flow cytometric analysis (10,000 events/sample) was performed on a Fortessa Flow Cytometer (BD Biosciences) with the CellQuest software (BD Biosciences), to quantify T cell activation using the FlowJo software (FlowJo, Ashland, OR, USA). Absolute quantification of each cell was generated by the number of viable cells, which was measured by cell counter, multiplied by the percentage of each item. The percentage of each item was determined by flow cytometry with a flow of 10,000 cells after isolation of PBMC.
Statistical methods
Data were analysed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (12). The Wilcoxon signed rank sum test was used to compare pre- and post-operative values for each anaesthesia (volatile desflurane, volatile sevoflurane, and intravenous propofol). Repeated measures ANOVA was used for comparison among the three groups at any given point. Post-hoc test was performed with Greenhouse-Geisser correction Hynh-Feldt correction after repeated measures ANOVA.
P<0.05 was considered statistically significant; results are presented as the mean ± SD.
Results
Eighty-two patients were included in this study between June 13, 2018 and July 24, 2019). Recruitment was discontinued at the achievement of scheduled numbers of samples; benign diseases and missing samples were excluded. Samples from 64 individuals (20 in group D, 22 in group S, and 22 in the group P) were analysed (Figure 1); their clinical characteristics are summarised in Table 1. There were no differences regarding age, sex, BMI, ASA physical status, WHO tumour stage, surgery time, separated lung ventilation time, and hospital stay among the three groups. Pre- and post-operative numbers of leukocyte and lymphocyte were also shown in Table 1. Leukocyte values among three groups were significantly different at both points, however the values of lymphocyte were not different among three groups at either point.
Table 1
| Characteristics | Desflurane | Sevoflurane | Propofol | P value |
|---|---|---|---|---|
| Number of patients | 20 | 22 | 22 | |
| Age (years), mean ± SD | 69.2±8.9 | 69.0±9.0 | 68.8±8.9 | 0.993 |
| Male, n (%) | 15 (75.0) | 15 (68.2) | 15 (68.2) | 0.858 |
| BMI, mean ± SD | 22.5±2.8 | 22.8 ±4.2 | 24.5±3.6 | 0.149 |
| ASA physical status, n (%) | 0.625 | |||
| 1 | 0 (0.0) | 2 (9.1) | 2 (9.1) | |
| 2 | 14 (70.0) | 16 (72.7) | 14 (63.6) | |
| 3 | 6 (30.0) | 4 (18.2) | 6 (27.3) | |
| WHO tumor stage, n (%) | 0.48 | |||
| 0 | 3 (15.0) | 2 (9.1) | 1 (4.5) | |
| I | 9 (45.0) | 12 (54.5) | 17 (77.3) | |
| II | 4 (20.0) | 5 (22.7) | 1 (4.5) | |
| III | 3 (15.0) | 1 (4.5) | 2 (9.1) | |
| Metastatic tumor | 1 (5.0) | 2 (9.1) | 1 (4.5) | |
| Surgical duration | 148.0 [81.0–215.0] | 155.0 [35.0–240.0] | 146.5 [83.0–245.0] | 0.984 |
| Anesthetic duration | 190.5 [128.0–282.0] | 202.0 [73.0–295.0] | 188.5 [119.0–304.0] | 0.978 |
| Duration of One lung ventilation | 134.5 [54.0–206.0] | 125.0 [27.0–220.0] | 131.0 [70.0–224.0] | 0.987 |
| Hospital stay after surgery, day | 5.0 [3.0–59.0] | 6.0 [3.0–18.0] | 5.0 [4.0–27.0] | 0.293 |
| Surgical procedure, n (%) | ||||
| Lobectomy | 14 (70.0) | 16 (72.7) | 19 (86.4) | 0.449 |
| Segment resection | 2 (10.0) | 4 (18.2) | 2 (9.1) | |
| Partial resection | 4 (20.0) | 2 (9.1) | 1 (4.5) | |
| Histological type, n (%) | ||||
| Adenocarcinoma | 16 (80.0) | 15 (68.2) | 14 (63.6) | 0.551 |
| Squamous cell carcinoma | 3 (15.0) | 3 (13.6) | 7 (31.8) | |
| Small cell carcinoma | 0 (0.0) | 1 (4.5) | 0 (0.0) | |
| Sarcoma | 0 (0.0) | 1 (4.5) | 0 (0.0) | |
| Metastatic tumor | 1 (5.0) | 2 (9.1) | 1 (4.5) | |
| Leukocyte (109/L) | ||||
| Preoperative | 6.20 [4.00–8.70] | 5.10 [2.90–8.20] | 5.85 [3.30–11.60] | 0.029 |
| Postoperative | 6.75 [4.10–12.80] | 4.85 [2.20–14.80] | 5.80 [2.90–13.80] | 0.048 |
| Lymphocyte (%) | ||||
| Preoperative | 25.90 [11.80–46.60] | 32.20 [16.50–46.20] | 30.55 [15.30–44.40] | 0.513 |
| Postoperative | 19.20 [10.30–38.30] | 23.50 [7.00–44.70] | 22.55 [6.70–38.60] | 0.666 |
The data are presented as mean ± SD, median [range], or number (percentage). ASA, American Society of Anesthesiologists.
Propofol decreased the number of CD8+ T cells in patients after lung surgery
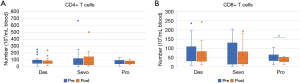
There was no difference in the number of CD4+ T cells and the ratio of PD-1 on CD4+ and CD8+ cells in the three groups; however, in group P, the number of CD8+ T cells was significantly lower after the operation than before [50.4±23.2 to 37.5±12.9 (104/mL blood), P<0.05]. In groups S and D, the number of CD8+ T cells did not decrease after the operation (Figure 2).
Sevoflurane increased proportion of regulatory T cells in patients after lung surgery
In group S, the proportion of regulatory T cells had significantly increased after surgery, compared with before (0.0086%±0.0062% to 0.012%±0.0079%, P<0.05, Figure 3). In groups P and D, the proportion of regulatory T cells did not increase after the operation.

Proportion of PD-1 in CD4+ and CD8+ T cells after lung surgery using propofol, sevoflurane, and desflurane
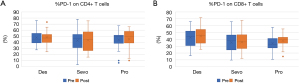
To investigate the precise mechanism of immunosuppression caused by anaesthesia, we identified expressed PD-1 in peripheral T cells in patients after surgery; there was no difference in PD-1 on CD4+ and CD8+ T cells after lung surgery using propofol, sevoflurane, and desflurane (Figure 4). These results suggest that propofol and sevoflurane may cause immunosuppression after lung cancer surgery by different mechanisms. The pre- and post-operative changes for all parameters were not significantly different among the three groups.
Discussion
This study revealed that propofol decreased the number of CD8+ T cells. It may lead to a diminished immune function against cancer since CD8+ T cell differentiates into cytotoxic T lymphocyte (CTL), which attacks cancer cells. This study also revealed that sevoflurane increased the proportion of regulatory T cells in patients after lung surgery. It may lead to suppression of the immune response against cancer cells since regulatory T cells suppress the activation of CTL and promote its death. However, propofol, sevoflurane, and desflurane did not increase the proportion of PD-1 on CD4+ and CD8+ T cells after lung surgery.
Naive CD8+ T cells recognise MHC class 1 dendritic cells, expressing IL-2 receptors. CTLs enter the tumour site and perform their functions, but the tumour microenvironment suppresses the transport and function of CTLs by chemokine secretion, abnormal tumour angiogenesis, and activation of inhibitory checkpoints (13). In the current experiment, propofol reduced CD8+ T cells; however, desflurane and sevoflurane did not cause a significant reduction. The reduction of CD8+ T cells by an anaesthetic leads to a further decrease in cytotoxic activity against tumours. Our results differ from those of other reports, according to which sevoflurane reduces CD8+ cells (9).
Regulatory T cells regulate the immune response to the self in the periphery, and have been implicated in immune escape in cancer (14). Regulatory T cells accumulate in the tumour bed of non-small cell lung cancer, suppressing cytotoxic antitumor elements and creating a favourable environment for tumour growth (5). While it has been reported that CD39 and CD73 on regulatory T cells induce adenosine formation and promote cancer progression, there was no difference in the frequency of their expression on regulatory T cells between use of sevoflurane and propofol (15). In our study, there was no significant increase in regulatory T cells with propofol and desflurane; however, there was a significant increase in regulatory T cells with sevoflurane. These results suggests that sevoflurane may contribute to cancer progression through an increase in regulatory T cells.
PD-1 is expressed on the surface of CTLs. PD-1 ligands, PD-L1 and PD-L2, are expressed on the surface of antigen-presenting cells. When CTLs infiltrate tumour tissue, cytokines such as IFN-γ secreted by CTLs cause cancer cells and tumour-infiltrating macrophages to express PD-L1 and PD-L2, which bind to PD-1 and inhibit CTL activity (16). PD-1 expression is increased in patients with sepsis and has been shown to be closely associated with hospital infection and mortality (17,18). Several studies showed increased level of PD-1 on T cells in cancer patients, such as Cervical Intraepithelial Neoplasia (19), in non-small cell lung cancer (NSCLC) (20). Tumour-infiltrating CD8+ T cells in NSCLC showed increased PD-1 expression and decreased immune function, including decreased cytokine production and proliferative capacity (21). There are reports showing that NSCLC patients have a relatively high content of PD-1+ CD4+ T cells, and that a decrease in PD1+ CD8+ T cells after cancer vaccination (PPV) correlates with survival (22). These results indicate that the baseline of PD-1 expression on T cells is higher than non-cancer patients, leading to our result that propofol, sevoflurane, and desflurane did not increase the proportion of PD-1 on CD4+ and CD8+ T cells after lung surgery. If other immune check points were investigated, the change depending on the anesthetics might have been observed. We are going to plan the measures of other checkpoints in the next study.
This study has several limitations. First, it is a single-centre study; thus, it may have been influenced by several biases, including patient characteristics, surgical manipulation, and experimentation techniques. Second, this study evaluated only the number of cells, and did not examine cellular functions such as cytokine production capacity. Third, the effects of analgesics on immunity were not assessed in this study. Opioids can be affected by intravenous or volatile anaesthetics of any choice, while reports suggest that morphine may promote cancer growth in lung cancer (23). Although opioids have an immunosuppressive effect, pain stress is also thought to be involved in immunosuppression; the balance between the two is difficult to quantify and assess (24). Since we intraoperatively used opioids such as fentanyl and morphine, which have known immunosuppressive effects, we should evaluate and consider these effects. Fourth, the effect of surgical manipulation on immunity could not be excluded. A variety of factors have been implicated in the peri-operative immune status, including the effects of surgical stress on the hypothalamus-pituitary-adrenal gland, direct effects on the sympathetic nervous system, and hypothermia (25); the T cell system, as measured in this experiment, may also be affected by these effects. Fifth, various immune cells have been found to infiltrate the tumour bed and participate in cancer growth (5). The immune cells might be affected by the released cancer cell to systemic blood flow by surgical manipulation; however, it is difficult to distinguish this from the other effects on immune cells. We intend to further investigate this in mice without cancer, to evaluate the specific effects of anaesthetics on immune cells.
Conclusions
Propofol decreased the number of CD8+ T cells, while sevoflurane increased the proportion of regulatory T cells in patients after lung surgery. However, propofol, sevoflurane, and desflurane did not increase the proportion of PD-1 on CD4+ and CD8+ T cells after lung surgery. Therefore, propofol and sevoflurane may be related to the immunosuppression in lung cancer patients. Future research in this direction is necessary.
Acknowledgments
Funding: This research was supported by the Grant-in-Aid for Scientific Research (KAKENHI) of The Ministry of Education, Culture, Sports, Science and Technology (MEXT) (18 K08865).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-878
Trial Protocol: Available at https://dx.doi.org/10.21037/jtd-21-878
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-878
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-878
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-878). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institution ethics board of Juntendo University Hospital (No. 17-286) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Pawelec G. Immunosenescence and cancer. Biogerontology 2017;18:717-21. [Crossref] [PubMed]
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. [Crossref] [PubMed]
- Gregg R, Smith CM, Clark FJ, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol 2005;140:540-6. [Crossref] [PubMed]
- O'Callaghan DS, O'Donnell D, O'Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024-36. [Crossref] [PubMed]
- Wigmore TJ, Mohammed K, Jhanji S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 2016;124:69-79. [Crossref] [PubMed]
- Enlund M, Berglund A, Andreasson K, et al. The choice of anaesthetic--sevoflurane or propofol--and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci 2014;119:251-61. [Crossref] [PubMed]
- Buckley A, McQuaid S, Johnson P, et al. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth 2014;113:i56-62. [Crossref] [PubMed]
- Schneemilch CE, Ittenson A, Ansorge S, et al. Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J Clin Anesth 2005;17:517-27. [Crossref] [PubMed]
- Yoo S, Lee HB, Han W, et al. Total Intravenous Anesthesia versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology 2019;130:31-40. [Crossref] [PubMed]
- Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 2003;97:1331-9. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in Tumor Microenvironment. Comput Struct Biotechnol J 2019;17:1-13. [Crossref] [PubMed]
- Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol 2016;28:401-9. [Crossref] [PubMed]
- Oh CS, Lee J, Yoon TG, et al. Effect of Equipotent Doses of Propofol versus Sevoflurane Anesthesia on Regulatory T Cells after Breast Cancer Surgery. Anesthesiology 2018;129:921-31. [Crossref] [PubMed]
- He J, Hu Y, Hu M, et al. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci Rep 2015;5:13110. [Crossref] [PubMed]
- Guignant C, Lepape A, Huang X, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 2011;15:R99. [Crossref] [PubMed]
- Zhang Y, Li J, Lou J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care 2011;15:R70. [Crossref] [PubMed]
- Chen Z, Pang N, Du R, et al. Elevated Expression of Programmed Death-1 and Programmed Death Ligand-1 Negatively Regulates Immune Response against Cervical Cancer Cells. Mediators Inflamm 2016;2016:6891482 [Crossref] [PubMed]
- Zheng H, Liu X, Zhang J, et al. Expression of PD-1 on CD4+ T cells in peripheral blood associates with poor clinical outcome in non-small cell lung cancer. Oncotarget 2016;7:56233-40. [Crossref] [PubMed]
- Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol 2010;7:389-95. [Crossref] [PubMed]
- Waki K, Yamada T, Yoshiyama K, et al. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci 2014;105:1229-35. [Crossref] [PubMed]
- Fujioka N, Nguyen J, Chen C, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg 2011;113:1353-64. [Crossref] [PubMed]
- Ogawa K, Hirai M, Katsube T, et al. Suppression of cellular immunity by surgical stress. Surgery 2000;127:329-36. [Crossref] [PubMed]
- Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth 2008;22:263-77. [Crossref] [PubMed]